- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
CMS Needs to Address Medicare Underfunding in 2017 Hospital Inpatient Rule for Bone Marrow Transplantation
The Be The Match Registry has seen phenomenal success with bone marrow and umbilical cord transplants. After having overcome donor availability, it is now important for CMS to create standardized reimbursement policies for the procedure.
Blood Cancers and the Evolution of Hematopoietic Stem Cell Transplants
For years, cancer easily outpaced scientific progress. However, we are finally pulling even with blood cancers. Every 3 minutes, a diagnosis of blood cancer changes a life forever; every 9 minutes, a life is lost to the disease. This may seem bleak, but there is hope: hematopoietic stem cell transplants can cure blood cancers and multiple nonmalignant diseases.1 For a sense of scale, there are more than 1 million people here in the United States living with, or in remission from, lymphoma, myeloma, or leukemia.
Hematopoietic progenitor cells (HPCs) are known as the “parent” cells from which all other blood cells develop. HPCs are found in blood and bone marrow, but these cells are often too damaged from chemo and radiation or they continue to manifest the underlying disease.2 HPC transplants are used to replace or rebuild a patient’s hematopoietic system. Patients who undergo HPC transplants may also experience graft-versus-tumor effect, eliminating residual disease. Put simply, HPC transplants often function as the only therapy with curative intent for patients with 1 of the more than 70 kinds of blood cancers and other blood disorders (such as leukemia, lymphoma, and myelodysplastic dysplasia), and they are becoming increasingly successful with each passing year.
Of the many reasons for improved outcomes with HPC transplantation, better matching of recipient to donor has had significant impact. Only 30% of patients have a perfect match among their siblings; in the past, the best outcomes for others were managed through transplant using an unrelated adult donor or with umbilical cord blood. With the growth of the world’s adult donor registries to nearly 30 million individuals, many more patients have been able to find an acceptable unrelated donor. On this front, sheer altruism has helped turn the tide against cancer. Additionally, with advances to control graft-versus-host disease in settings in which human leukocyte antigens (HLAs) are less than perfectly matched between donor and recipient, the use of half-matched family members (haploidentical transplants) is improving access for thousands of patients who otherwise might not be eligible for curative therapy.3
It’s worth expanding on this point. Just 3 decades ago, Congress created a predecessor to the C.W. “Bill” Young Cell Transplantation Program in order to establish a national registry of adult volunteer donors and of publicly available cord blood units. During this period, our nation’s Be The Match Registry ballooned to more than 16 million adult volunteer marrow donors and 238,000 cord blood units. After factoring in international relationships, the global donor base includes approximately 29 million potential marrow donors and 712,000 cord blood units. Numbers have translated to action. Be The Match facilitated nearly 6200 marrow and umbilical cord blood transplants in 2016, for a total of 80,000 transplants since 1987.4
The takeaway here is simple: it’s no longer a lack of donors that prevents life-saving transplantations. On the contrary, factors like the flawed federal payment policies are what now most directly limit America’s transplantation infrastructure. While this is discouraging, some CMS policymakers have ignited the engine of reform in the outpatient setting,5 according to the most recent Hospital Outpatient Prospective Payment System (HOPPS) rule. More importantly, it takes the action of only a few decision makers in Washington to push forward even more meaningful change.
The Intersection of Care and Medicare
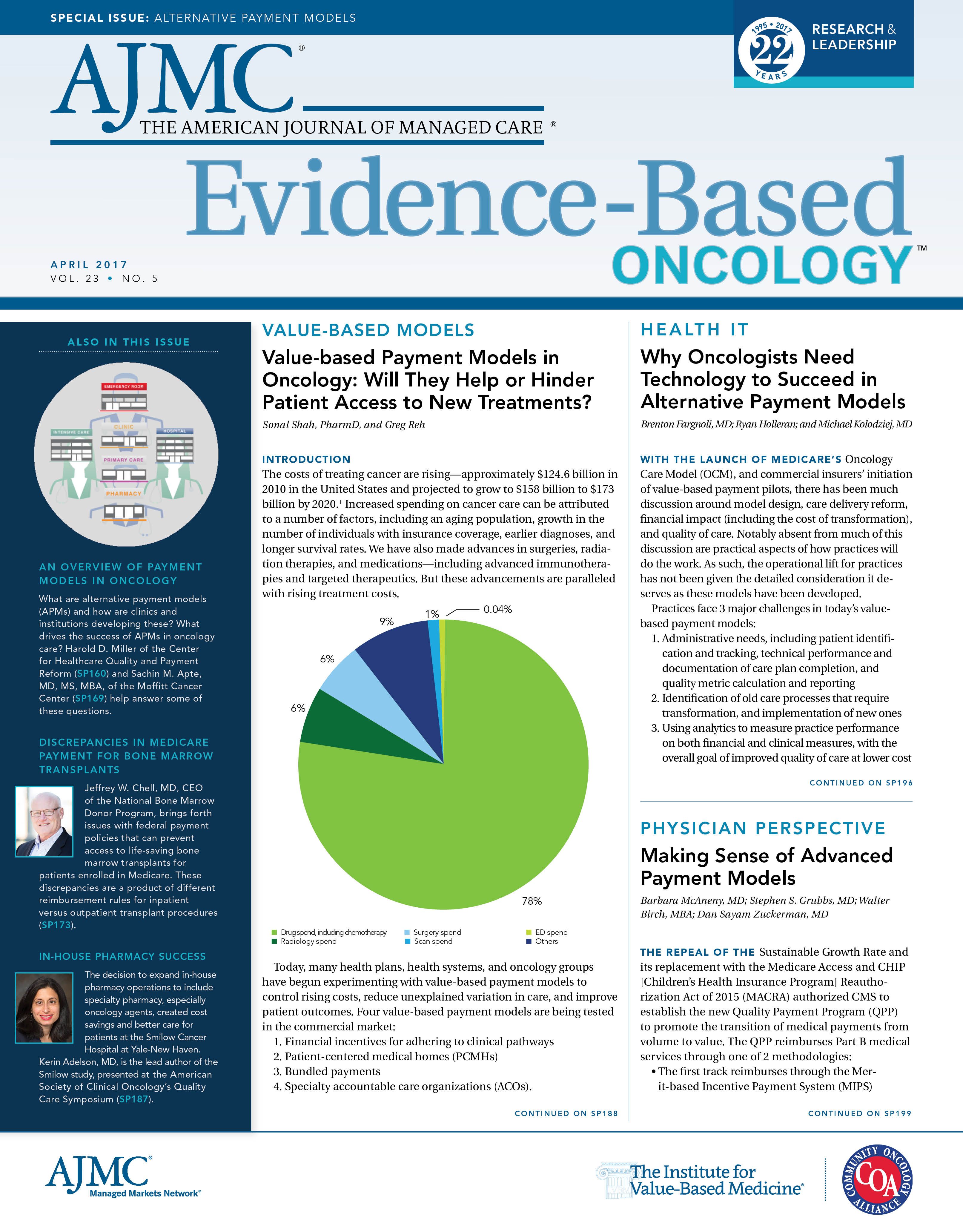
In order to understand the problem at hand, it’s important to delve into the intricacies of Medicare payment policy. Every year, CMS puts forth a HOPPS rule as well as a Medicare Inpatient Prospective Payment System (IPPS) rule. As can be expected, the HOPPS rule pertains to procedures performed on patients who do not require hospital admission, while the IPPS rule applies to patients who require prolonged monitoring.
For years, both the HOPPS and the IPPS rules reimbursed significantly below the cost of HPC transplantation. In the outpatient setting, for example, the federal reimbursement rate was a stunning 47% below the procedure’s true cost.6 This shortfall manifested itself, in part, because CMS’ payment formulae did not account for the cost of the marrow or cord blood acquisition. These procurement costs are anything but insignificant: the cost of locating and transporting HPCs to a patient in need frequently exceeds $45,000. As a result, many hospitals performing bone marrow transplants for Medicare patients regularly report losing tens of thousands of dollars on each case. Unsurprisingly, this triggered significant access issues, as both outpatient and inpatient facilities were hemorrhaging funds.
Logically, the chasm between Medicare reimbursement rates and the actual cost of care does not make much sense. So why is such a strategy in place? Although there is no overt explanation, it is very likely that federal policymakers—especially those who originally drafted the IPPS and HOPPS rules—did not anticipate that individuals over 65 years would benefit from marrow and stem cell transplants, as pretransplant treatment regimens were poorly tolerated by older patients with comorbid disease. Before 2000, many transplant programs considered being older than 50 years as a contraindication for a transplant from an unrelated donor. While this may have been appropriate years ago, today, the median age of diagnosis for acute myeloid leukemia, one of the most prominent blood cancers, is 67 years, according to the National Cancer Institute.7 Moreover, Medicare beneficiaries are the most rapidly growing age segment for transplantation, receiving nearly 1100 procedures in 2015 alone (Figure 1). Most importantly, however, the shift toward an older demographic has arisen alongside improved clinical outcomes. HPC transplants are saving lives for those over 65 years, delivering hope where hope had not previously existed.
For these very reasons, the vast majority of private insurers cover 100% of the expenses for patients over 65 years who need HPC transplants. To veterans of health policy, this scenario will seem backwards. Typically, the government bears the burden of expensive, yet necessary, procedures while private payers drag their feet. However, when it comes to life-saving marrow and stem cell transplants, it has been the exact opposite. That’s right: The government agency most responsible for the health and well-being of the American people has, for years, looked the other way.
Rewriting HOPPS for the Better
Fortunately, the CMS/HHS team responsible for HOPPS finally took notice, and on November 1, 2016, CMS laid out its final HOPPS rule that would increase reimbursement to address the current inadequate rates that did not cover outpatient treatment costs, including the cost of acquiring bone marrow and cord blood for transplant.
The new rule contains significant changes to the payment amount and methodology for reporting costs related to bone marrow and cord blood transplants, which limits the use of the outpatient setting for transplant due to the significant underpayment under the current methodology. Key aspects of the rule include:
1. Outpatient hematopoietic cell transplantation (HCT; Current Procedural Terminology code 38240) will be moved into a new Comprehensive Ambulatory Payment Classification (C-APC). This allows all of the costs submitted on an outpatient HCT claim to remain together and be averaged with other outpatient HCT claims versus being diluted by other lower-cost services in a broader, noncomprehensive APC.
2. Payment for the new C-APC is proposed to be $27,752. This is a significant increase from the 2016 rate of $3015 and the proposed rate of $15,267. Although this still does not reflect the total acquisition costs associated with unrelated allogeneic transplant, let alone other costs incurred as part of the outpatient procedure, the new C-APC methodology will allow for upward adjustment based on cost reporting practices.
3. CMS has finalized a new cost center line for tracking donor procurement and related charges: new standard cost center 77, “Allogeneic Stem Cell Acquisition.” Currently, donor-related costs are within a more general revenue code, which was subject to a cost:charge ratio edit based upon broader blood products data. By having a dedicated revenue code, CMS will have a clearer understanding of these costs and will better adjust rates in the future. This will apply only to allogeneic HCT.
4. Acquisition charges, including National Bone Marrow Donor Program fees and costs of HLA typing, donor evaluation, and collection of cells, among other costs, will be specifically required to be reported in Field 42 on CMS Form 1450 (UB-04) so that CMS may assess the charges and gauge how well the C-APC payment reflects the costs of providing these services.
While there remains room for improvement in the reimbursement rate to pay for cell acquisition costs, for which transplant centers are currently under reimbursed, the new methodology is unquestionably a step in the right direction. However, 1 major problem is that most transplants do not occur in outpatient facilities; rather, the vast majority of HPC procedures take place in the inpatient setting. Further, a lack of coordination between federal policymakers dealing with HOPPS and IPPS has prevented government efforts to harmonize standards. Therefore, precarious payment and access issues continue to persist.
Inaction for Inpatients
Not only do 90% of all HPC transplants take place in the inpatient setting, but the reimbursement deficits exceed those of outpatient facilities. On this front, the numbers are notable. As illustrated in Figure 2, the Medicare base reimbursement rate was surpassed by average hospital organ acquisition charges in 2015,8 which means that Medicare has not historically paid enough to cover the costs of procurement. Hence, each additional dollar that the hospital must bill for the transplantation itself contributes to a net loss. Cost-reimbursement deviations are even greater at the state level. In Georgia, for instance, hospitals performing cord blood transplants are already $10,652 in the hole even before admitting a patient. This number is higher in places like Rhode Island, where inpatient facilities are $21,540 in the red before treatment begins. Still, this begs the question: how much does a transplant typically cost?
The actual scale of unreimbursed expenses is significant. As illustrated in Figure 3, the average reported hospital charge for an HPC transplant in 2015 was $399,019, while the Medicare Severity Diagnosis Related Group (DRG) 14 base reimbursement was $64,452—a difference of $334,567.8 Those managing hospital finances would rightfully scoff at such an imbalance. Despite being potentially detrimental for patient access, many medical facilities are understandably assessing the sustainability of performing future transplants. Such uncertainty, however, could be eliminated via common-sense, comprehensive action, as was done with HOPPS.
Addressing IPPS Underfunding
CMS officials managing the inpatient payments can implement 2 policy solutions:
- Rewrite the IPPS rule to raise the base Medicare reimbursement rate, which is fairly straightforward. In the outpatient setting, policymakers lifted reimbursement rates by a factor of 9 in 2016 alone. Hence, there is every reason to believe that such convincing action can be mirrored.
- There is also a strategic workaround available to those in Washington: to reimburse cellular transplants in the same manner as solid organs (eg, kidneys). Under current regulations, Medicare provides a type of pass-through for acquisition costs, reimbursing hospitals for these costs separate from the IPPS rate. In this way, the government guarantees that hospitals will be adequately compensated for acquisition expenses and that such expenses do not create a disincentive for providing transplants to older patients. Implementing a policy similar to that for living kidney donors would not entail a massive overhaul of federal policies, but simply recognizing the acquisition costs apart from the DRG, as is done with solid organs. The solution makes sense on multiple levels, as it would create parity across Medicare transplant policies and reduce the role of cost in limiting access for beneficiaries.
Moreover, such a policy would have a positive impact on patients, while making an insignificant dent in Medicare spending. As shown in Figures 4 and 5, HCTs cost less than both cornea and kidney transplants and they are needed by fewer patients.9 Therefore, it just makes sense for CMS to reimburse hospitals for their cell acquisition cost separate from the DRG rate, just as they do for the acquisition cost of solid organs.
ACKNOWLEDGMENTS:
None.
FUNDING SOURCE:
None.
Jeffrey W. Chell, MD, is chief executive officer of National Marrow Donor Program.
ADDRESS FOR CORRESPONDENCE
Jeffrey W. Chell, MD
CEO, National Bone Marrow Donor Program
500 N 5th St.
Minneapolis, MN 55401-1206
E-mail: jchell@nmdp.org.
In the end, the future is brighter than ever before for patients suffering from blood cancers. Technology is progressing rapidly, medical treatments are tackling diseases that were death sentences just decades ago, and policymaking is finally beginning to catch up with this progress. We now need CMS to take the next logical step and create standardized, fair reimbursement rules for all Medicare beneficiaries, no matter where they choose to receive care. As a physician and an advocate, I will echo the same message I have delivered so often: the evidence is clear, and it is time for a change. It’s what my patients and so many others deserve.REFERENCES
1. Facts and statistics. Leukemia & Lymphoma Society website. https://www.lls.org/http%3A/llsorg. prod.acquia-sites.com/facts-and-statistics/facts-and-statistics-overview/facts-and-statistics. Accessed February 20, 2017.
2. Hematopoietic progenitor cell transplant. Ministry Health Care website. http://ministryhealth. org/Services/Cancer/Locations/SaintJosephsHospital/Transplant/HematopoieticProgenitorCell- Transplant.nws. Accessed February 20, 2017.
3. Haploidentical stem cell transplant. Leukemia Foundation website. http://www.leukaemia. org.au/treatments/stem-cell-transplants/haploidentical-stem-cell-transplant/haploidentical- stem-cell-transplant. Accessed February 20, 2017.
4. Internal data. Be The Match.
5. Details for title: CMS-1656-FC. Hospital outpatient prospective payment—final rule with comment and final CY2017 payment rates. CMS website. https://www.cms.gov/Medicare/Medicare- Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices- Items/CMS-1656-FC.html. Accessed February 20, 2017.
6. Majhail NS, Mau LW, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone Marrow Transplant. 2013;48(2):294-300. doi: 10.1038/bmt.2012.133.
7. Cancer Stat Facts: acute myeloid leukemia (AML). National Cancer Institute website. http://seer. cancer.gov/statfacts/html/amyl.html. Accessed February 20, 2017.
8. Medicare Provider and Analysis Review Database, 2015; Centers for Medicare & Medicaid Services.
9. Bentley TS. 2014 U.S. organ and tissue transplant cost estimates and discussion. Milliman website. http://www.milliman.com/uploadedFiles/insight/Research/health-rr/1938HDP_20141230. pdf. Published December 2014. Accessed February 20, 2017.
Ambient AI Tool Adoption in US Hospitals and Associated Factors
January 27th 2026Nearly two-thirds of hospitals using Epic have adopted ambient artificial intelligence (AI), with higher uptake among larger, not-for-profit hospitals and those with higher workload and stronger financial performance.
Read More

