
- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Navigating Decision-Making When Treating Lymphoma With Bispecific Antibodies vs CAR T-Cell Therapy
Todd Wiseman was getting anxious. The Olathe, Kansas–based musician and construction worker had been through 18 weeks of chemotherapy to manage his diffuse large B-cell lymphoma (DLBCL) only to have the disease surge back a month later. His physician recommended a chimeric antigen receptor (CAR) T-cell therapy, axicabtagene ciloleucel, or axi-cel (Yescarta; Kite Pharma). Wiseman, who had spent more than 4 decades recording, performing, producing, and teaching guitar while more recently picking up construction projects for house flippers, had understood the therapy to be a potentially one-and-done durable treatment for his aggressive disease.
However, the first part of the process, where a patient’s cells are collected and genetically engineered to target the patient’s cancer, wasn’t successful. Wiseman’s cells didn’t convert to CAR T cells, a rare situation known as a failure to manufacture that only occurs in 1% of patients, according to data shared in an email from the University of Kansas Medical Center in Kansas City. Wiseman repeated the 2-week step only to have the manufacturing process fail a second time.
“At that point I was pretty desperate and wondering, ‘Well, what’s up now?’” Wiseman said in an interview with Evidence-Based Oncology.
That’s when his physician, Nausheen Ahmed, MD, assistant professor of hematologic malignancies and cellular therapeutics at The University of Kansas Cancer Center in Kansas City, suggested a different approach. Instead of modifying his own cells, Wiseman could participate in a clinical trial that would provide so-called off-the-shelf allogeneic T cells, or donor T cells, which wouldn’t require weeks to collect, transform, and infuse back into his body.
He agreed and received 2 infusions of CTX110, a CD19-directed T-cell immunotherapy comprised of allogeneic T cells genetically modified ex vivo using CRISPR-Cas9 gene editing components (NCT04035434).1
“So we did that, and that was a decent experience,” Wiseman said. “It wasn’t really hard on my body or anything compared with other things.”
Still, the therapy failed to keep his disease at bay for long. “It did not kill the lymphoma completely, but it set me up to where I was in good enough shape to do a stem cell transplant. And so that was my procedure.” In early September, Wiseman passed his 190-day posttransplant checkup, with no signs of the lymphoma returning.
Although oncologists readily praise CAR T-cell treatments as revolutionary for their potential to send stubborn cancers into remission for long periods and even eradicate the disease completely, they are not without their drawbacks and complications, as experienced by Wiseman and others.
These medications, which can run up to $1 million when hospital costs are included,2 can take weeks, if not months, to make—assuming a patient can first secure an appointment to collect their blood and separate the white blood cells to send to the manufacturer, a process called leukapheresis. Then, the manufacturer, who also needs to have equipment availability, engineers and expands the patient’s white blood cells into CAR T cells. The manufactured therapy is sent back to the hospital, where it is administered via infusion to the patient. Post infusion, the patient must be monitored closely for potential adverse effects, which can require hospital stays for days while physicians look for signs of a toxic immune response. Finally, negotiating with insurers to pay not just for the therapy itself but for those hospital stays as well can delay the start of treatment.
Enter bispecific antibodies. These therapies, which bind to T-cell receptors in the patient and drag them to the cancer cells, are newer to the market than CAR T cells for DLBCL but more accessible. Instead of weeks, patients can typically start off-the-shelf therapy within days. They’re cheaper as well, making them more attractive to budget-conscious hospital systems. However, bispecific antibodies require regular infusions, tying patients to repeated hospital visits for hours at a time. And their long-term effectiveness and durability are unknown.
Further complicating matters is what sequence patients should receive these treatments. In some cases, their bodies may experience T-cell exhaustion and not respond to whichever therapy is given after the first one, Michael Tees, MD, said. Tees is a physician at the Colorado Blood Cancer Institute in Denver and part of the Lymphoid and Autoimmune Disease Groups. Because CAR T cells have been available longer, they are approved for earlier lines of treatment than bispecific antibodies. But patients who have failed 3 or more rounds of therapy could be eligible for either one.
So what’s an oncologist to do?
“This is a great problem for us to have,” Jason Westin, MD, director of the Lymphoma Clinical Research Program and section chief of the aggressive lymphoma research team in the Department of Lymphoma and Myeloma at the University of Texas MD Anderson Cancer Center in Houston, said in an interview. “Having 2 home run therapies and figuring out the right way to sequence them or combine them in the future, this is our great challenge. But we’re very glad to have this problem with multiple great therapies to help people in need.”
MD Anderson Cancer Center has treated more than 1000 patients with CAR T cells since their approval in 2017 and “maybe in the low double digits” with bispecifics since their approval earlier this year for DLBCL, Westin said.
“At the end of the day, if a patient has access to more than 1 option for treatment, what’s going to be the main driving factor is which is most likely to help them get into remission, to hopefully cure them of their disease and avoid potentially dying from the relapse of their disease,” Westin said. “Thankfully, both CAR T cells and bispecifics are highly effective treatments.”
The first CAR T-cell therapy to be approved3 by the FDA was tisagenlecleucel (Kymriah; Novartis) in August 2017, followed by axi-cel in October of that year.4 The agency has approved5 4 additional CAR T-cell treatments since then for conditions including follicular lymphoma, refractory mantle cell lymphoma, B-cell acute lymphoblastic leukemia, B-cell non-Hodgkin lymphoma, and multiple myeloma (Table 1). In the past year, the regulatory body expanded axi-cel’s use to second-line DLBCL,6 fueling a 29% year-over-year surge in the drug’s sales to $380 million for manufacturer Gilead’s Kite Pharma, according to their second-quarter earnings report issued August 3, 2023.7
Table 1.
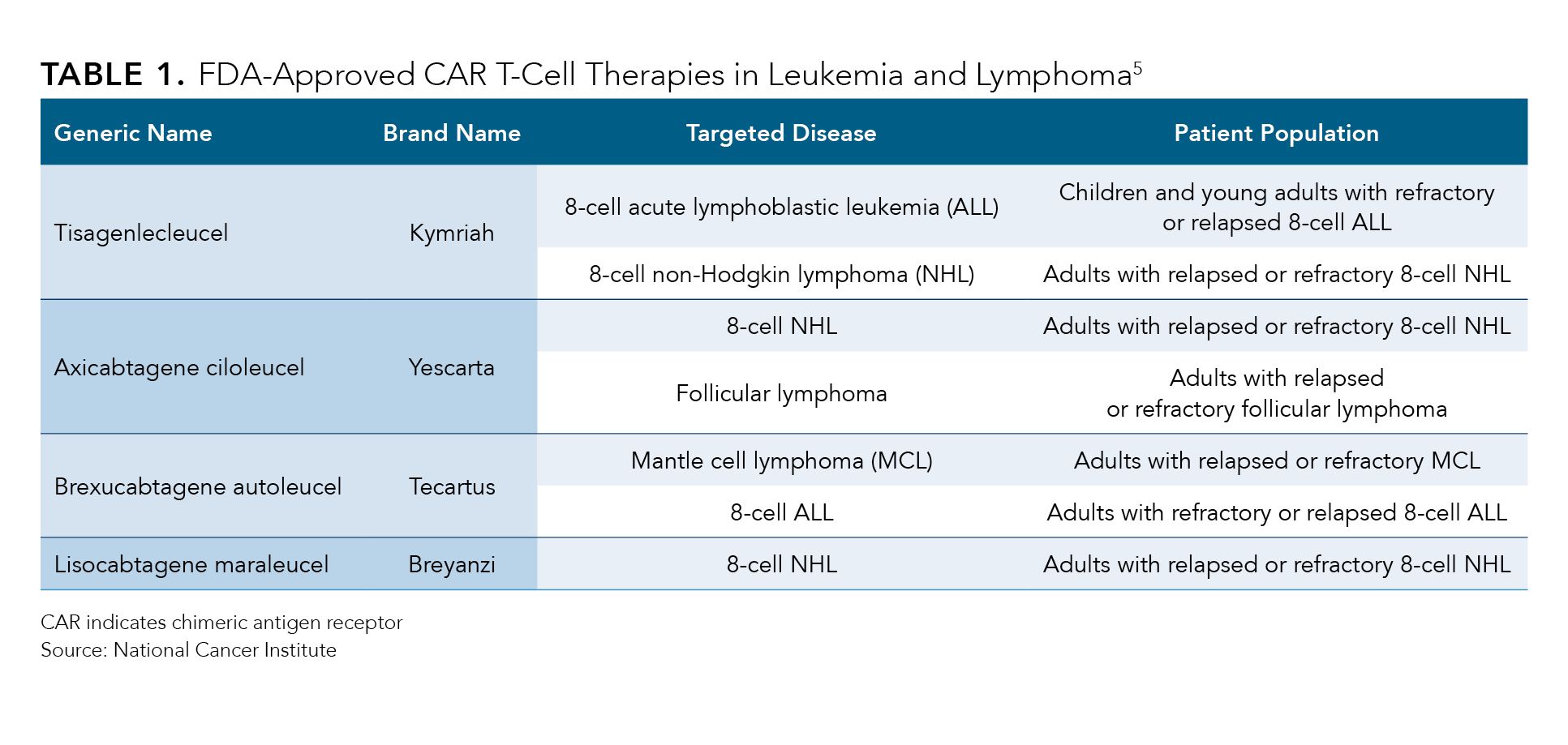
“Overall, Yescarta is the first treatment in nearly 30 years to demonstrate a significant improvement in survival for this patient population,” Merdad Parsey, MD, PhD, chief medical officer at Gilead, said during the earnings call, according to a transcript.7 These data add to the growing body of evidence that cell therapy is potentially “curative in some populations,” he added.
Axi-cel competes with Bristol Myers Squibb (BMS)’s CAR T-cell therapy lisocabtagene maraleucel (Breyanzi), which the FDA first approved8 in February 2021 for third-line use in DLBCL and expanded9 to a second-line treatment in June 2022. Sales of lisocabtagene maraleucel have more than doubled from the past year to $100 million, driven by the earlier treatment line and increased manufacturing capacity, David Elkins, BMS’ chief financial officer, said during the company’s second-quarter earnings call on July 27, 2023, according to a transcript.10
On the bispecifics side, the first therapy to win FDA approval was blinatumomab (Blincyto) in 2014 for Philadelphia chromosome–negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The agency has signed off11 on 8 additional bispecific antibodies, including 2 approvals this year: AbbVie and Genmab's epcoritamab-bysp12 (Epkinly) and Roche-owned Genentech’s glofitamab-gxbm13 (Columvi), both as third-line treatments for DLBCL (Table 2).
Table 2.
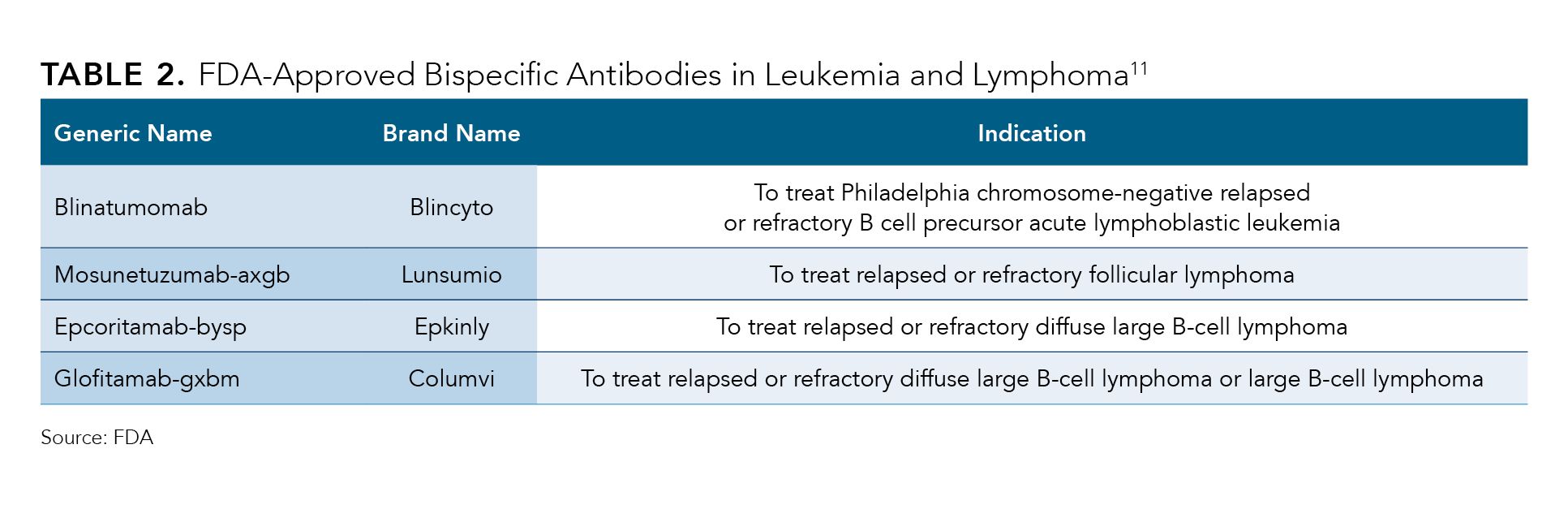
Roche’s Genentech bispecific antibody is unique in that although other treatments in this class require indefinite infusions, glofitamab is administered for 13 infusions over the course of approximately 8.5 months.14 Charles Fuchs, senior vice president and global head of Oncology and Hematology Drug Development at Roche, said during the company’s second-quarter earnings call on July 27, 2023, that even some patients who had failed a CAR T-cell treatment saw benefit from glofitamab.
“Columvi provides the potential best-in-class therapy, with off-the-shelf efficacy that is comparable with CAR T [cells] for patients with diffuse large B-cell lymphoma,” Fuchs said during the earnings call, according to a transcript.15 “The data continue to show clinically meaningful outcomes in heavily pretreated refractory patients with diffuse large B-cell lymphoma, including those who had progressed or recurred after CAR T-[cell] therapy, with a complete response rate of 40% and a median duration of complete response of roughly 27 months.”
The Leukemia & Lymphoma Society’s chief medical officer, Gwen Nichols, MD, said the time crunch to get CAR T cells can be an issue.
“A lot of the issues with CAR T [cells] have to do with how long it takes to manufacture,” she said in an interview. “Right now, even if you’re at [Memorial] Sloan Kettering [Cancer Center in New York, New York] and you’ve got CAR T [cells] down the hallway, there’s still a time pressure, and if the patient’s disease is galloping off, you won’t have time.”
Both Gilead’s Kite Pharma and BMS have emphasized existing and future plans to speed production time for their CAR T cells and expand manufacturing capacity, because some physicians have called out a lack of availability or long wait times for some of these cell therapies as a bottleneck.16
Kite Pharma claims to have an industry-leading 16-day median time from leukapheresis to product release, with a 96% manufacturing success rate.17 Chris McDonald, senior vice president and global head of technical of operations at Kite, credits the quick turnaround to his organization’s advanced planning to build manufacturing capacity and secure the raw materials needed to create CAR T cells, which can cause issues for other manufacturers.
“We really have dialed in the entire supply chain and the ability to order raw materials, manufacture the product, test the product, release the product, and get the product back to the patient as quick as possible,” he said in an interview. “We’ve seen that turnaround time really has a significant impact on patient outcomes.”
With CAR T cells now being the standard of care for second- and third-line patients with DLBCL, the challenge remains in getting patients to the right treatment centers so they can receive the medicine in a timely manner, Joseph P. McGuirk, DO, said. McGuirk is the Schutte-Speas Professor of Hematologic Malignancies and Cellular Therapeutics at the University of Kansas Medical Center in Kansas City. He estimates 60% of patients who receive a diagnosis of DLBCL are cured with their first line of treatment, leaving 40% potentially eligible for a CAR T-cell therapy. However, McGuirk said that during the treatments’ first full year on the market in 2018, only approximately 700 patients out of 6400 eligible patients ended up receiving a CAR T-cell therapy (a 2023 review18 led by fellow University of Kansas Cancer Center hematologist-oncologist Marc S. Hoffman, MD, discussed various reasons for the low referral rate).
“That’s woefully inadequate,” McGuirk said. Although the situation has somewhat improved as the therapy has become the standard of care in second-line treatment, only approximately 30% of eligible patients are receiving CAR T cells, he said. “Seventy percent never come to us. We don’t even have a conversation, and they’re not referred. So that’s abysmal, and it’s a national problem. It’s a national tragedy in my opinion.”
Another issue that can arise is a delay in approval from insurance companies, adding weeks to the process, McGuirk said. “And a couple of weeks can represent critical and life-threatening time frames for patients in need with large cell lymphoma.”
Where a patient lives can also determine whether CAR T cells would be an appropriate treatment, Nichols adds.
“If you’re 200 miles away, it’s a big deal; you’ve got to go multiple times to the facility to get cells harvested, and that can be a major undertaking,” Nichols said. “So there are a lot of things that get in the way of being able to successfully obtain CAR T-[cell] therapy.”
For this reason, she sees the use of bispecific antibodies increasing. “Because bispecifics are off the shelf, there’s going to be slowly but surely more uptake in clinics that aren’t in giant cancer centers,” Nichols said. “I can talk to a patient this afternoon and set up for them to get the bispecific tomorrow in the clinic.”
For Frederick W. Locke, MD, an oncologist and translational researcher in the Department of Blood and Marrow Transplant and Cellular Immunotherapy at Moffitt Cancer Center in Tampa, Florida, referrals from community physicians and hospitals that don’t provide CAR T cells to larger centers such as his own can present a barrier to treatment.
For its part, Gilead’s Kite Pharma has a team focused on outreach to community oncologists, including independent grants to educate about the need for earlier patient identification and referrals for CAR T-cell therapy, according to the company.
“There are some reimbursement issues that make it difficult to give this therapy at certain hospitals or centers,” Locke said. “Primarily because there’s a bill not only for the associated care, for providing CAR T-cell therapy and managing it and managing the toxicities, there’s a bill due for the product itself.”
Locke said the Moffitt Cancer Center has treated more than 300 patients with cell therapies, with mostly CAR T-cell therapy for lymphoma and multiple myeloma. As a high-volume center, Moffitt Cancer Center has ample access to manufacturing slots and a large team to work with patients, payers, referring physicians, and manufacturers to ensure the process progresses as smoothly as possible.
“CAR T-cell therapy is clearly revolutionary. We have patients who are getting remissions, deep and durable remissions, even cures in some diseases,” Locke said. “My belief is that cell therapy and CAR T-cell therapy offer superior outcomes for patients compared with bispecific antibodies, acknowledging that bispecifics do allow for an easier use, and that potentially in the future we will have allogeneic donor-derived CAR T cells, which will mitigate some of those problems with CAR T cells and allow us to give it to more patients.”
Managing toxicities from CAR T cells could make some physicians who don’t regularly guide patients through the treatment wary of the therapy, Westin said.
“It is potentially an anxiety point for treating physicians who have never done this before,” Westin said. “To feel like you have a handle on it, that page comes in at 3 in the morning that the patient’s having a problem, that you know how and what to do for that.”
To help clinicians outside of MD Anderson Cancer Center better manage potential adverse effects from CAR T cells, the organization launched a mobile app19 in 2018 called CARTOX. The free app incorporates MD Anderson Cancer Center’s Immune Effector Cell Therapy Toxicity Assessment and Management guidelines.
“I believe it has been a helpful tool for clinicians managing the potential adverse effects of immune effector cell therapy,” Sherry Adkins, the nurse practitioner at MD Anderson Cancer Center who helped to develop the app, said in an email. “It has been downloaded over 46,000 times and in 21 countries.”
Tees said his organization has been able to use remote patient monitoring to free up resources to treat more patients with CAR T cells.
Tees looked at his center’s data as well as data from published studies on when patients undergoing CAR T-cell therapy were experiencing toxicities and saw that they mostly occur 5 to 10 days after infusion. Instead of keeping patients in the hospital for a week or more, the team moved to an outpatient-first model, allowing patients to have their vital signs checked every 30 minutes remotely from the comfort of their home and only bringing them in if signs of toxicity arose.
“We have been super successful with this, which has allowed us to continue doing what we’re doing as these [bispecific antibodies] get approved,” Tees said. “This has allowed us to not shut the door to the outside referring doctors for the patients who need to get this care because they can’t do it.”
Westin, McGuirk, Locke, and Tees all credit their organizations’ robust support teams in ensuring the process goes smoothly and quickly as possible. Factors include helping patients with travel to the hospital center, engaging with insurers early and often to avoid approval delays, and making sure manufacturing slots are available for patients.
“All the logistics are very important, but at the bottom level, the most critical thing is how effective is the treatment,” Westin said. “And thankfully, both CAR T cells and bispecifics are highly effective treatments.”
References
1. A safety and efficacy study evaluating CTX110 in subjects with relapsed or refractory B-cell malignancies (CARBON). ClinicalTrials.gov. August 18, 2023. Accessed September 14, 2023. https://www.clinicaltrials.gov/study/NCT04035434
2. Real-world CAR-T treatment costs can range from $700,000 to $1 million. News release. Prime Therapeutics. April 1, 2021. Accessed September 25, 2023. https://bit.ly/48kHQQS
3. FDA approval brings first gene therapy to the United States. News release. FDA. August 30, 2017. Accessed September 14, 2023. http://bit.ly/3sQi32N
4. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. News release. FDA. October 18, 2017. Accessed September 25, 2023. https://bit.ly/456MuPM
5. CAR T cells: engineering patients’ immune cells to treat their cancers. National Cancer Institute. Updated March 10, 2022. Accessed September 11, 2023. https://bit.ly/3ZpVQEX
6. FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma. FDA. April 1, 2022. Accessed September 25, 2023. https://bit.ly/3RqnXzB
7. Gilead Sciences (GILD) Q2 2023 Earnings Call. August 3, 2023. Accessed September 14, 2023. Transcript available at: https://bit.ly/3PBo8ZM
8. FDA approves new treatment for adults with relapsed or refractory large-B-cell lymphoma. News release. FDA. February 5, 2021. Accessed September 14, 2023. https://bit.ly/48dzN8t
9. U.S. FDA approves Bristol Myers Squibb’s CAR T cell therapy Breyanzi for relapsed or refractory large B-cell lymphoma after one prior therapy. News release. Bristol Myers Squibb. June 24, 2022. Accessed September 14, 2023. https://bit.ly/3sNGnCi
10. Bristol Myers Squibb (BMY) Q2 2023 Earnings Call. July 27, 2023. Accessed September 14, 2023. Transcript available at: https://bit.ly/3ZknG5w
11. Bispecific antibodies: an area of research and clinical applications. FDA. August 2, 2023. Accessed September 14, 2023. https://bit.ly/3Pm8PTB
12. FDA grants accelerated approval to epcoritamab-bysp for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma. FDA. May 19, 2023. Accessed September 14, 2023. https://bit.ly/3EEmkcf
13. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. FDA. June 16, 2023. Accessed September 14, 2023. https://bit.ly/44NWM73
14. Columvi fact sheet. Genentech. Accessed September 14, 2023. https://bit.ly/3PhzfWs
15. Roche Holding AG (RHHBY) Q2 2023 Earnings Call. July 27, 2023. Accessed September 28, 2023. Transcript available at: https://seekingalpha.com/article/4620674-roche-holding-ag-rhhby-q2-2023-earnings-call-transcript
16. Caffrey M. Sequencing, sustainability are hot topics in CAR T- and gene cell therapies discussion. Am J Manag Care. 2023;29(3):SP221.
17. Kite receives U.S. FDA approval of new state-of-the-art CAR T-cell therapy manufacturing facility in Maryland. News release. Gilead. April 19, 2022. Accessed September 14, 2023. https://bit.ly/48dAS01
18. Hoffman MS, Hunter BD, Cobb PW, Varela JC, Munoz J. Overcoming barriers to referral for chimeric antigen receptor T cell therapy in patients with relapsed/refractory diffuse large B-cell lymphoma. Transplant Cell Ther. 2023;29(7):440-448. https://doi.org/10.1016/j.jtct.2023.04.003
19. MD Anderson releases free CAR T-cell therapy toxicity grading and management app. Healio. June 7, 2019. Accessed September 14, 2023. https://bit.ly/3REfhZ1

Addressing Financial Toxicity With Navigators and Better Conversations Around Decision Making
June 5th 2018Financial toxicity impacts a lot more than a patient's finances—it leads to nonadherence and poorer health outcomes. Financial navigators are increasingly being used to be proactive about addressing high healthcare costs and the resulting financial toxicity.
Listen
Addressing Financial Toxicity With Navigators and Better Conversations Around Decision Making
June 5th 2018Financial toxicity impacts a lot more than a patient's finances—it leads to nonadherence and poorer health outcomes. Financial navigators are increasingly being used to be proactive about addressing high healthcare costs and the resulting financial toxicity.
Listen
2 Commerce Drive
Cranbury, NJ 08512
AJMC®
All rights reserved.
