- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Evaluating Label Warnings, Switching Rates in Patients With Psoriasis Initiating New Treatment
Posters presented at the 2023 Fall Clinical Dermatology Conference evaluated how label warnings may affect new treatment initiation and real-word switching patterns for patients starting risankizumab.
Two posters presented at the 2023 Fall Clinical Dermatology Conference aimed to evaluate trends in how label warnings and precautions may affect the proportion of patients with psoriasis initiating new treatments and identify real-world switching rates for patients with psoriasis initiating risankizumab.
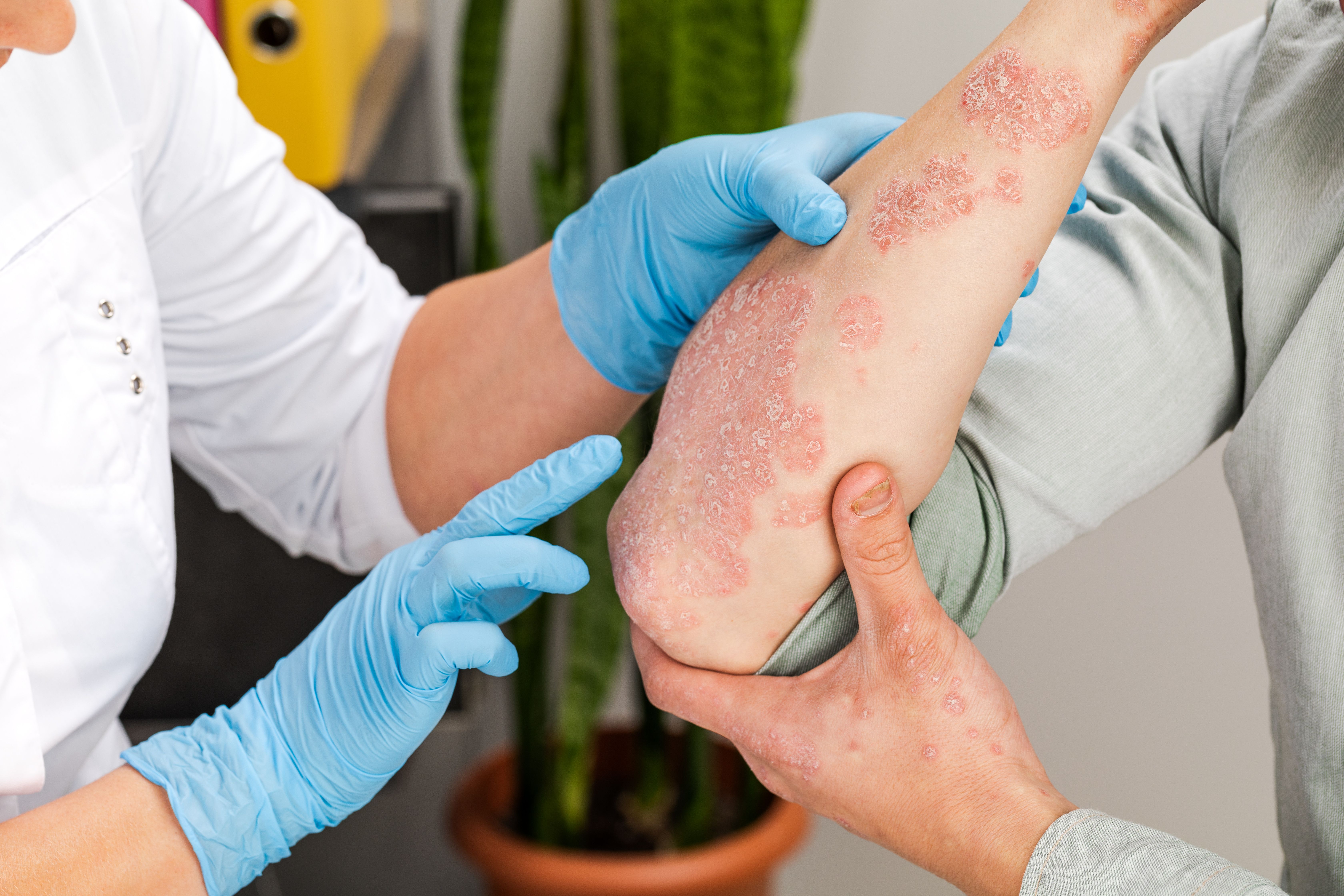
When initiating or switching treatments, it is important that patients and their physicians consider factors such as disease severity, quality of life, comorbid conditions, and treatment history. Additionally, warnings and labels are important factors to consider, as these warnings often include malignancy, risk of infection inclusive of immunosuppression, etc.
The first retrospective observational study assessed the proportion of patients with psoriasis in the United States seeking systemic treatment who may have been impacted by label warnings and precautions between September 2017 and September 2022.1
Baseline characteristics of age, sex, region, comorbidities, and treatments were assessed 365 days prior to initiation. Additionally, categories related to label warnings were evaluated at the initiation of a new treatment, and these were immunosuppressive medications, infections, malignancies, and any category.
A total of 125,096 new treatment initiations were identified during the study period. The mean (SD) patient age was 58.2 (15.4) years and 54.5% were female patients.
The proportion of patients with malignancies tended to be older, with a mean age (SD) of 68.1 (11.4) years. Although more patients with psoriasis had commercial insurance, those meeting the label-warning and precaution criteria were more likely to have Medicare. Forty-three percent reporting having Medicare overall, 65.9% reported being on immunosuppressants, 58.7% reported infections, 69.7% reported malignancies, and 61% fit any label warning category.
Furthermore, 26.3% of patients initiating a new systemic treatment met the criteria needed for that treatment, according to warning labels and precautions. Of these group, 9.4% were immunosuppressed, 16.1% had recent infections, and 5.2% had active malignancies. These findings support the consideration of these factors when beginning a new treatment, the authors noted.
The second study used an insurance claims database linked to electronic medical records (EMRs) to identify real-world switching rates for patients with psoriasis treated with risankizumab, stratified by body mass index (BMI) and weight quartiles.2
Patients included in this second study had at least 1 psoriasis diagnosis according to International Classification of Diseases codes prior to or on the date of initiation, had 6 or more months of continuous insurance benefits pre- and 12 months post biologic initiation, had no other biologics or apremilast during the baseline period, and had weight/height data available on or 12 months prior to the start of treatment.
A total of 367 patients were included in the analysis, and their mean age was 47.7 (14.3) years, 52.9% were female patients, 5.5% were Black patients, and 83.1% were White patients.
Of these patients initiating risankizumab, 19.4% had a BMI below 25 kg/m2, 30.2% had a BMI of 25 to less than 30 kg/m2, and 50.4% had a BMI of 30 kg/m2 and higher. Furthermore, each body weight stratified group included approximately 25% of the patient population.
Additionally, the switch rate at 12 months for all patients initiating risankizumab was 3.8%, with no significant differences identified between BMI categories (P = .589) or body weight quartiles (P = .299).
Therefore, these findings suggest that treatment patterns at 12 months of initiating risankizumab remained consistent regardless of BMI or body weight quartile.
References
1. Langley R, Jardon S, Montgomery J, et al. How label warnings and precautions may impact the proportion of patients with psoriasis initiating new treatments. Poster presented at: 2023 Fall Clinical Dermatology; October 19-22, 2023; Las Vegas, Nevada.
2. Wu J, Patel M, Photowala H, et al. Psoriasis initiating risankizumab stratified by body mass index. Poster presented at: 2023 Fall Clinical Dermatology; October 19-22, 2023; Las Vegas, Nevada.
