- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Contributor: New Jersey Has Highest Average In-Network Costs for COVID-19 Complex Hospitalizations
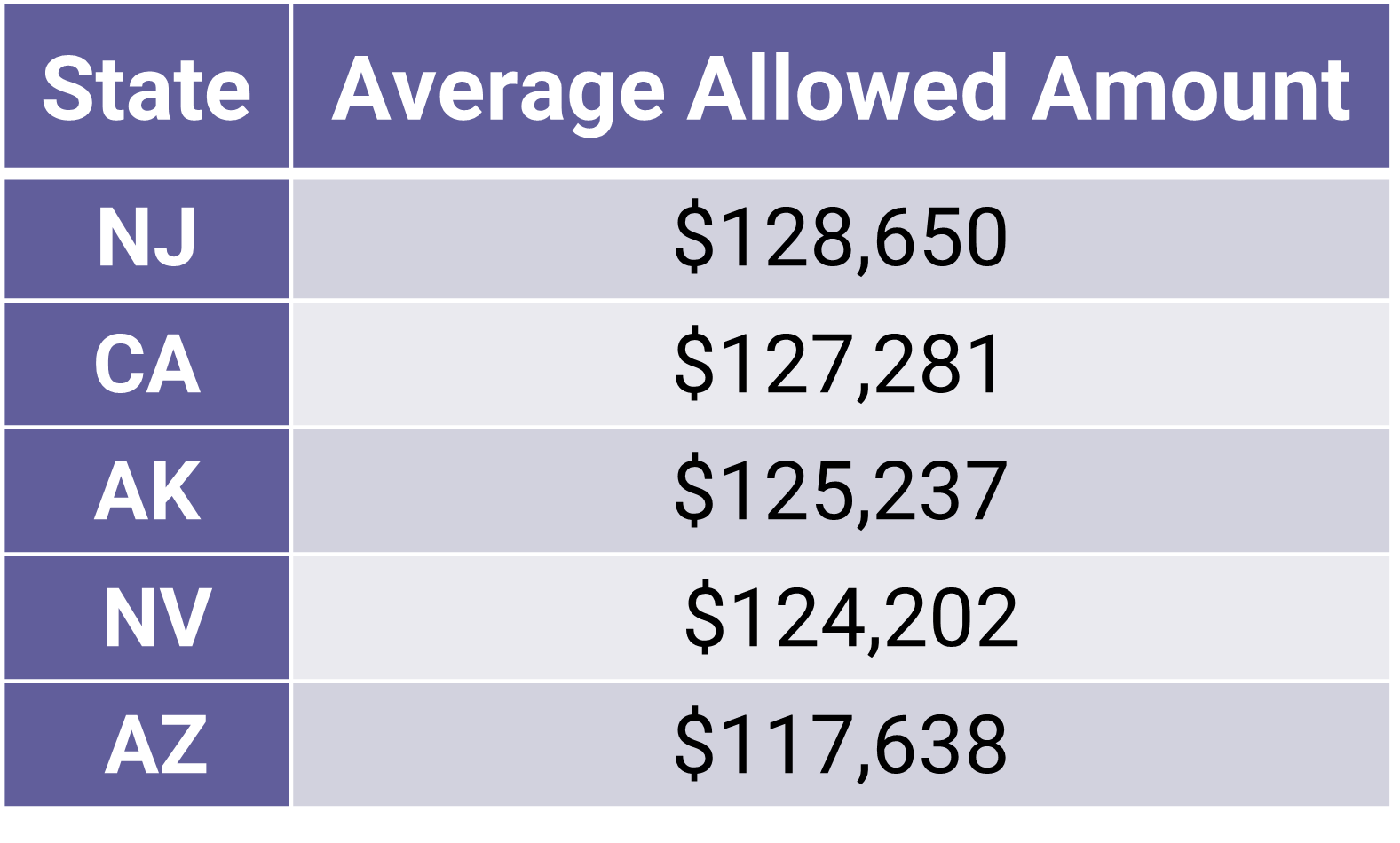
In a state-by-state analysis of private healthcare claims data from 2020 to 2021, New Jersey emerged as the state with the highest average allowed amount[1] for complex hospitalizations for COVID-19, while Maryland had the lowest. In New Jersey, the average allowed amount for such hospitalizations was $128,650 (Exhibit 1) and in Maryland $49,127. These and other findings are reported in the new FAIR Health brief COVID-19 Treatment and Hospitalization Costs: A Descriptive Analysis of the FAIR Health COVID-19 Cost Tracker. The brief provides a descriptive analysis of the COVID-19 patient population whose treatment and hospitalization costs are tracked by FAIR Health’s COVID-19 Cost Tracker, a free, online tool displaying typical, state-by-state COVID-19 costs.
Exhibit 1. Five States with the Highest Average Allowed Amounts for COVID-19 Complex Hospitalizations, 2020-2021
(Source: FAIR Health)
Complex hospitalizations for COVID-19 are those that require ventilation and/or admission to the intensive care unit (ICU), while noncomplex hospitalizations do not. The state with the highest average allowed amount for noncomplex hospitalizations for COVID-19 was Alaska ($44,239), and the state with the highest average allowed amount for outpatient treatment for COVID-19 was Nevada ($1,538). Maryland was the state with the lowest average allowed amounts for noncomplex hospitalizations ($12,531) and outpatient treatment ($580) for COVID-19, as well as for complex hospitalizations.[2]
Other findings in the new report include the following (from April 2020 to August 2021 unless otherwise indicated):
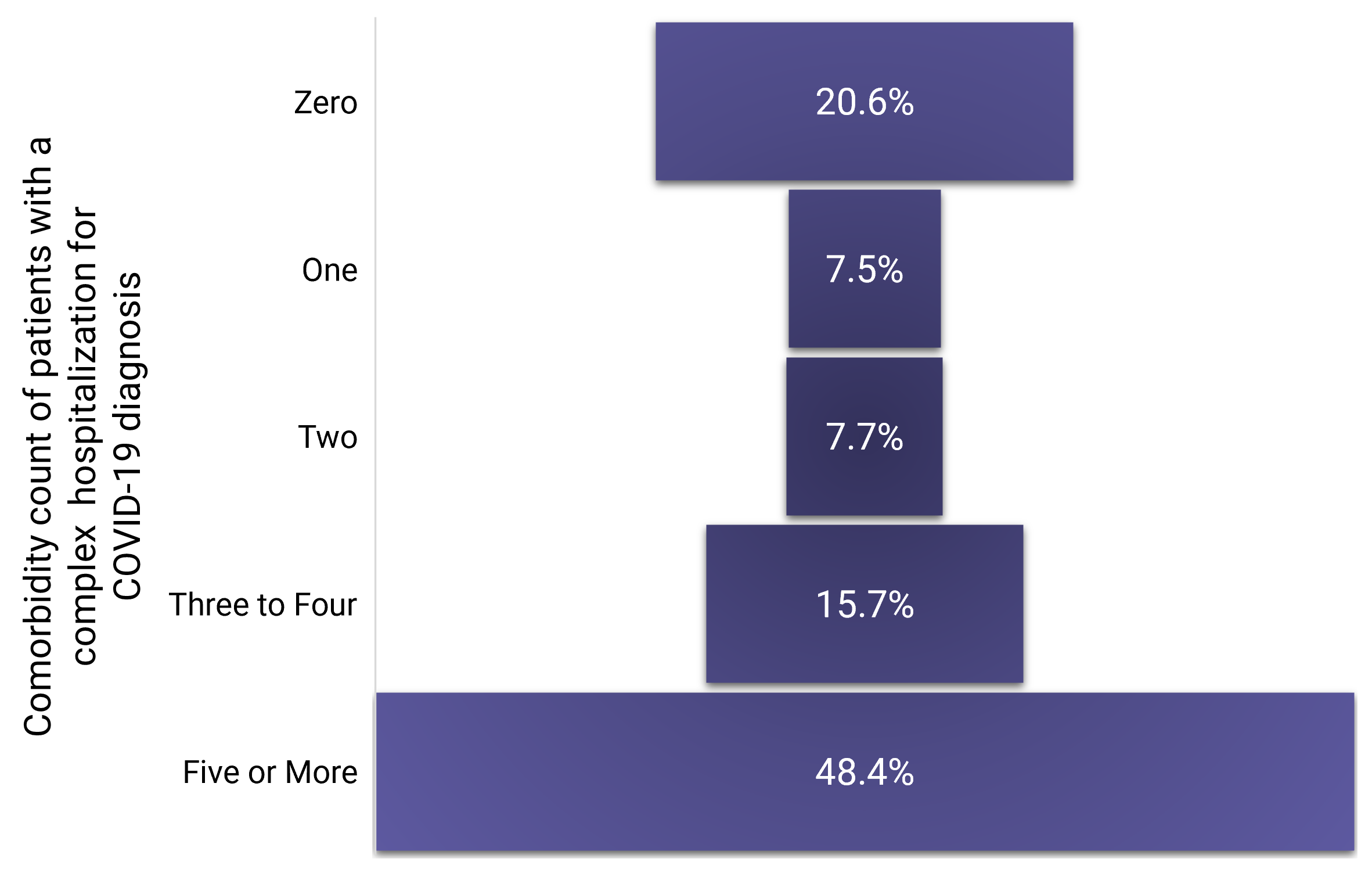
- Of COVID-19 patients with a complex inpatient stay, 48.4 percent had five or more comorbidities and 20.6 percent had zero comorbidities (Exhibit 2). By comparison, patients with zero comorbidities constituted nearly half (49.4 percent) of all patients diagnosed with COVID-19; patients with five or more comorbidities constituted only 13.7 percent of all patients diagnosed with COVID-19.
Exhibit 2. Distribution of Patients with a Complex Hospitalization for COVID-19 by Number of Comorbidities, April 2020 to August 2021
(Source: FAIR Health)
- In patients with a complex hospitalization for COVID-19, the most common comorbidity was hyperlipidemia and/or hypertension, which accounted for 14.7 percent of this population. In patients with a noncomplex hospitalization for COVID-19, the most common comorbidity was chronic breathing issues, at 6.5 percent of the distribution.
- December 2020 was the month with the most reported COVID-19 diagnoses.
- From January to June 2021, the distribution of COVID-19 diagnoses in urban areas was higher than in rural areas. But in July 2021, and even more in August 2021, rural areas had a greater distribution of COVID-19 cases than urban areas.
- The largest category of COVID-19 cases included those who tested positive for COVID-19 but did not receive any further services for COVID-related symptoms.[3] That category was larger than outpatients with symptoms, complex inpatients or noncomplex inpatients.
- The largest percentage of complex hospitalizations occurred in those 70 years and older (17.2 percent of patients diagnosed with COVID-19 in this age group); an additional 15.7 percent of patients in this age group had a noncomplex hospitalization. In total, 32.9 percent of all patients 70 years and older had an inpatient stay for their COVID-19 diagnosis.
- In noncomplex hospitalizations for COVID-19, 57 percent of patients were female, but in complex hospitalizations, 57 percent of patients were male.
- The percentage of COVID-19 patients who died in April 2020 was 1.9 percent, but from February to July 2021, it was about half a percent each month.
- The median length of stay for patients with a complex hospitalization for COVID-19 decreased from a high of 13 days in April 2020 to a low of 7 days in July 2021. The median length of stay for a noncomplex hospitalization, however, remained relatively flat throughout this period, with most months having a median of four days and the rest three days.
- For complex and noncomplex hospitalizations for COVID-19 in 2020 and 2021, the West had the highest average allowed amounts and the South the lowest. For outpatient treatment for COVID-19, the West had the highest average allowed amounts and the Northeast the lowest.
- Behind the numbers of our COVID-19 Cost Tracker are the individuals who have contracted COVID-19. As a public service, this brief provides a descriptive analysis of that patient population, offering context for the COVID-19 Cost Tracker.
This is the ninth in a series of studies released by FAIR Health on the COVID-19 pandemic. The first study examined projected US costs for COVID-19 patients requiring inpatient stays, the second the impact of the pandemic on hospitals and health systems, the third the impact on healthcare professionals, the fourth key characteristics of COVID-19 patients, the fifth the impact on the dental industry, the sixth risk factors for COVID-19 mortality, the seventh the impact on pediatric mental health and the eighth post-COVID conditions.
For the new brief, click here.
Robin Gelburd, JD, is the founding President of FAIR Health, a national, independent nonprofit organization that serves as a trusted leader in healthcare cost transparency, data analytics and benchmarks. FAIR Health possesses the nation’s largest collection of private healthcare claims data, which includes over 35 billion claim records and grows at a rate of over 2 billion claim records a year. Certified by the Centers for Medicare & Medicaid Services as a national Qualified Entity, FAIR Health also receives data representing the experience of all individuals enrolled in traditional Medicare Parts A, B and D. Robin is a nationally recognized expert on healthcare policy and health literacy and transparency. Selected as one of Crain’s 2019 Notable Women in Health Care, she has been invited to speak to organizations and federal and state agencies across the country and world.
[1] An allowed amount is the total fee negotiated between an insurance plan and a provider for an in-network service. It includes both the portion to be paid by the plan member and the portion to be paid by the plan.
[2] This may be partly explained by Maryland’s all-payer rate-setting system. See Maryland Hospital Association, “The Maryland Model.”
[3] Patients who did not report symptoms on the index date of diagnosis may have experienced symptoms afterward and not incurred a claim because they were not sick enough to seek medical care or did not choose to do so.
