- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Avoiding Surgical Resection of Recurrent BRAF V600E–Mutated Iodine-Refractory Papillary Thyroid Cancer Involving Trachea/Thyroid Cartilage via Resensitization With Dabrafenib and Trametinib: Report of 3 Cases
Introduction
The incidence of thyroid cancer has been increasing in recent years. Approximately 44,000 new cases are expected to be diagnosed in 2025, with a male-to-female ratio of 1:2.5.1 Papillary thyroid cancer (PTC), the most common subtype of well-differentiated thyroid cancer, has an excellent prognosis. However, approximately 50% of PTCs harbor a BRAF V600E mutation, associated with aggressive behavior and decreased 10-year survival.2 Up to 30% of patients with PTC have persistent or recurrent disease.2 Additionally, 50% of patients with metastatic differentiated thyroid cancer (DTC) are refractory to radioactive iodine (RAI) treatment.3 Iodine-refractory thyroid cancer (IRTC) portends a worse prognosis with a 10-year survival rate of just 10%.3
Recurrent IRTC invading the upper aerodigestive tract remains a formidable clinical challenge. Management typically involves tracheal resection or laryngectomy, often with sacrifice of the recurrent laryngeal nerve, and may include esophagectomy, followed by external beam radiation therapy (EBRT).4 This approach does not address the need to treat the distant metastasis, and it has a profound impact on quality of life, compromising essential functions such as speech, breathing, and swallowing while placing a significant burden on the patient’s psychosocial well-being.
We present 3 cases of recurrent BRAF V600E–mutated iodine-refractory PTC involving the trachea or thyroid cartilage in which dabrafenib/trametinib combination therapy cytoreduced and resensitized IRTC, allowing successful RAI therapy without surgical resection or EBRT. All patients have remained progression-free for at least 12 months without continued tyrosine kinase inhibitor (TKI) therapy.
Methods
Through chart review, we identified 3 patients with recurrent iodine-refractory BRAF V600E–mutated PTC involving the trachea or thyroid cartilage after initial total thyroidectomy, neck dissection, and RAI treatment. The investigators obtained informed consent to publish information and/or images from each participant. The clinical history and treatment course of each case are detailed below.
FIGURE.
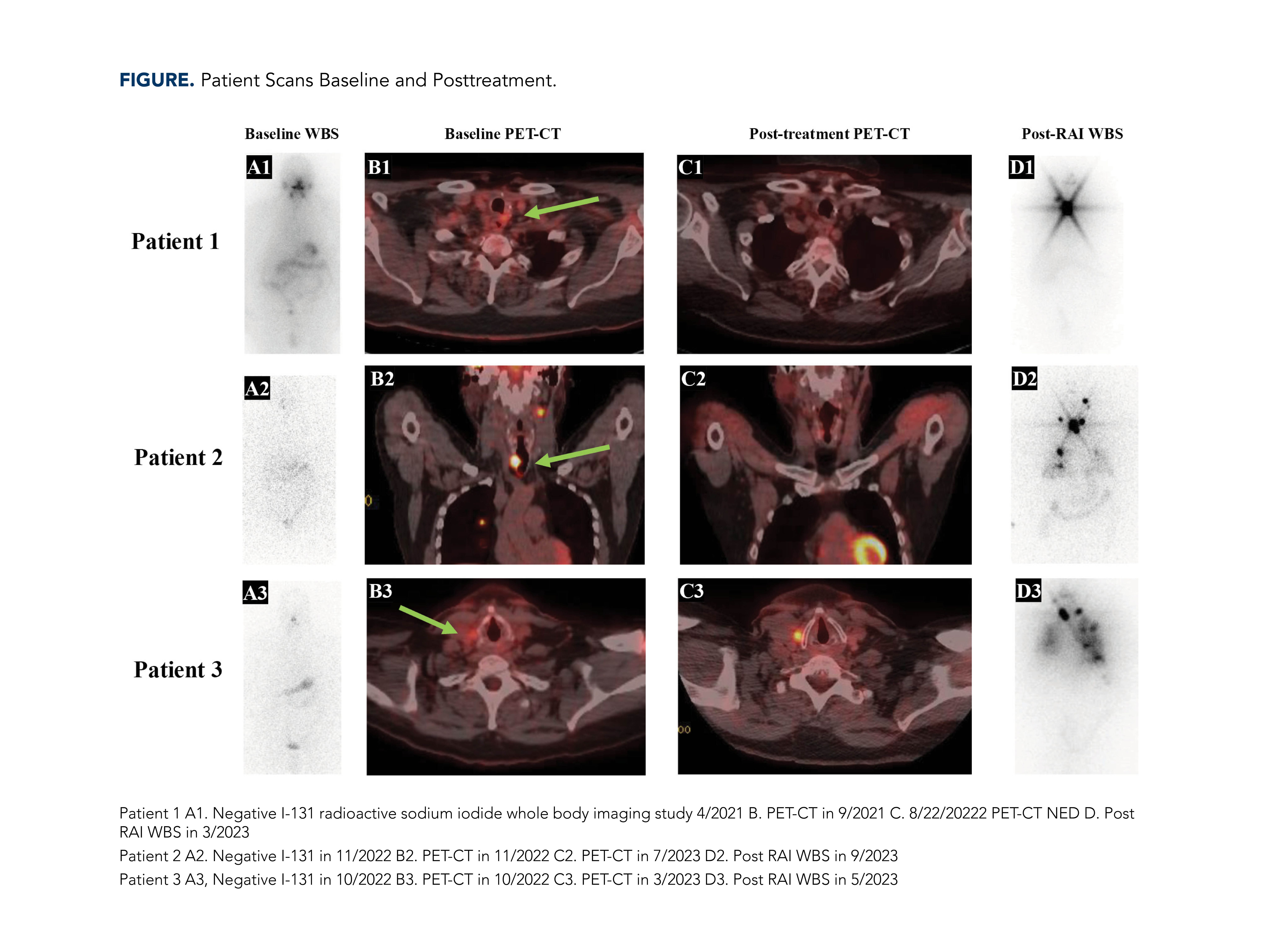
Cases
Patient 1
A 70-year-old White man was initially diagnosed with pT4apN1aM1 stage IVB PTC with lung metastasis. In January 2020, he underwent a total thyroidectomy with left central neck dissection and shave resection of a metastatic lesion in the left trachea. Pathology revealed a 4.7-cm PTC invading surrounding tissues, larynx, trachea, and esophagus, as well as metastasis in 3 level VI lymph nodes with extranodal extension.
He received RAI in March 2020. A whole-body scan (WBS) post ablation demonstrated uptake only in the thyroid bed. One year later, ultrasound detected a hypoechoic area on the lateral aspect of the left trachea. WBS in April 2021 was negative for uptake, indicating iodine refractoriness (Figure A1). Next-generation sequencing (NGS) revealed a BRAF V600E mutation. A PET-CT scan in September 2021 demonstrated fluorodeoxyglucose (FDG)–avid foci in the right lymph node and bilateral tracheoesophageal grooves (left focus is shown in Figure B1); the patient’s lung nodules were likely too small to show FDG avidity. Laryngoscopy also disclosed invasion of the cancer through the trachea. The case was discussed in multidisciplinary tumor board, and tracheal resection was not recommended due to the patient’s short neck and poor lung function.
The patient was started on TKIs with dabrafenib (Tafinlar)/trametinib (Mekinist) in November 2021 and had excellent tolerance. A PET/CT scan in August 2022 showed remarkable response without evidence of disease (Figure C1). The patient underwent successful RAI treatment (Figure D1) and discontinued dabrafenib/trametinib in March 2023. The patient has been progression-free for 18 months as of this writing.
Patient 2
A 47-year-old Native American man presented with pT4apN1bM1 stage II PTC with metastasis in the mediastinal lymph nodes and lungs. The patient underwent a total thyroidectomy with bilateral neck dissections in December 2021 and RAI in March 2022. By August 2022, the patient had an elevated thyroglobulin, and a CT scan in October 2022 demonstrated cancer recurrence with extension into the right tracheal wall and metastasis to the lateral neck lymph nodes and lungs, indicating clinically primary iodine-refractory disease. WBS in November 2022 exhibited no iodine uptake, confirming iodine-refractoriness (Figure A2). A PET/CT scan in December 2022 confirmed hypermetabolic disease in the bilateral neck and mediastinum, and innumerable lung lesions (Figure B2). NGS revealed a BRAF V600E mutation.
The patient experienced 2 episodes of hemoptysis in February 2023 due to tracheal invasion, which was confirmed by a laryngoscopy. The patient transferred to our center and received urgent combination therapy with dabrafenib/trametinib from March through September 2023. He responded rapidly, with resolution of hemoptysis within 2 weeks. After 6 months of treatment, the patient had near-complete response radiographically (Figure B3). The patient received RAI treatment in September 2023. Post-RAI WBS was positive, indicating successful resensitization. The patient has remained progression-free for 12 months without TKIs and has resumed full-time employment without any long-term complications or limitations.
Patient 3
A 60-year-old White man with cT2N1bM1 stage IVB PTC with lung metastasis underwent a total thyroidectomy and RAI therapy in 2016. Four months later, he had bilateral central neck dissections and right modified neck dissection, with 19 of 52 lymph nodes positive for metastasis. He received a second course of RAI in January 2017. Post-RAI WBS revealed only mild uptake in the neck, indicative of iodine-refractory disease.
In 2019, his physicians suspected that he had disease progression again in the cervical lymph nodes. The patient came to our center in 2022 for bilateral central neck dissections after a significant delay due to COVID-19. Nine of 9 positive lymph nodes were removed. However, an approximately 1-cm nodule invading the thyroid cartilage was left behind due to concern for pharyngeal/esophageal leak and the patient’s desire to avoid total laryngectomy. The patient was diagnosed with BRAF V600E–positive iodine-refractory PTC (Figure A3), with metastasis to the right thyroid cartilage, lung, left pleura, and second left rib (Figure B3). He was subsequently treated with dabrafenib/trametinib therapy for 7 months, which was complicated by severe hyponatremia, confusion, and muscle spasms requiring a dose reduction of dabrafenib. Interestingly, his posttreatment PET/CT scan showed increased FDG avidity in the nodule by the right thyroid cartilage, likely due to stimulation from thyrogen. The patient received RAI therapy with positive WBS post ablation, demonstrating successful resensitization. He has been treatment-free and event-free for 15 months.
Discussion
IRTC involving the aerodigestive tract is a challenging clinical scenario. Historically, surgical resection was the standard option. Without any valid systemic therapy, it is arguable that surgical resections could improve quality of life by preventing airway compromise, hemoptysis, or aspiration.
Better understanding of the molecular basis of thyroid cancer has led to the development of effective TKI therapy for IRTC. Multikinase inhibitors mainly targeting VEGFR (ie, sorafenib [Nexavar], lenvatinib Lenvima], cabozantinib [Cabometyx]) are now widely used in clinical practice.5 TKIs targeting RET, ALK, and NTRK have also recently become available.
The MAPK signaling pathway, involving BRAF and its downstream effector, MEK, is pivotal in thyroid cancer biology.6 BRAF V600E is a common gain-of-function mutation in many tumor types, and anti-BRAF/MEK combination therapy with dabrafenib/trametinib has been widely used to treat melanoma, non–small cell lung cancer, and anaplastic thyroid cancer harboring BRAF V600E.6 In a small, randomized phase 2 study comparing dabrafenib alone or in combination with trametinib in 53 patients with BRAF-mutant metastatic PTC, overall response rates were similar (42% vs 48%, respectively), but median progression-free survival (PFS) was longer in the combination group (10.7 vs 15.1 months).7 Currently, it is appropriate to use either dabrafenib alone or in combination with trametinib to treat BRAF V600E–mutant IRTC, although the National Comprehensive Cancer Network guideline has adopted combination therapy as either the frontline or second-line therapy in IRTC.8 The clinical application and long-term outcomes of this approach are still under intense clinical investigation.7,9-13
Although effective, TKI therapy does not cure IRTC. The median PFS is approximately 18 months for lenvatinib and 15 months for dabrafenib/trametinib.7 Moreover, TKIs can have significant adverse effects and are challenging to continue indefinitely, particularly in older patients.
RAI-R DTC is thought to develop from cellular dedifferentiation that causes loss of expression of the sodium-iodine symporter responsible for iodine uptake.4,9 The BRAF V600E mutation has been implicated in cellular dedifferentiation involved in thyroid cancers.9 It is postulated that targeting BRAF/MEK could redifferentiate (resensitize) iodine refractoriness. A few small studies have demonstrated that targeting either MEK alone or both BRAF/MEK for 4 to 6 weeks successfully resensitized patients with iodine-refractory, metastatic BRAF V600E IRTC, although combination therapy seemed more effective.10-13
Conclusion
We took advantage of a clinically available option targeting the BRAF V600E mutation in 3 patients with recurrent iodine-refractory PTC involving the trachea and/or thyroid cartilage. All 3 patients tolerated treatment reasonably well and responded remarkably to dabrafenib/trametinib combination therapy, as evidenced by PET/CT scans before and after treatment.
Because previous trial results have shown that administering TKIs for only 4 to 6 weeks has had limited success, with resensitization in less than 40% to 60% of patients, we purposely extended treatment to at least 6 months to resensitize iodine refractoriness and maximize cytoreduction.10-13 Abundant literature supports that smaller metastatic lesions, particularly those smaller than 1 cm in the lungs, respond more favorably to RAI therapy.14,15 Each of our 3 patients had excellent response to TKIs with optimal tumor size reduction, which contributed, at least in part, to the sustained benefit of RAI ablation after resensitization.
To our knowledge, this is the first report that dabrafenib/trametinib combination therapy was used successfully to treat BRAF-mutated IRTC recurrence involving the trachea or thyroid cartilage without surgical resection and indefinite TKI therapy. All 3 patients continue to experience event-free survival and excellent quality of life. This unique strategy highlights the therapeutic efficacy of TKIs in disease control and overcoming iodine refractoriness in this challenging clinical scenario. Further research is warranted to address this unmet clinical need.
Author Information
Melissa Papuc, MD, is a 2025 graduate of the University of Arizona College of Medicine Phoenix (UACOMP); Rosemarie Metzger, MD, is with the Division of Endocrine Surgery, Department of Surgery, UACOMP; Kresimira Milas, MD, is chief of the Division of Endocrine Surgery, Department of Surgery, UACOMP; Christian Nsar, MD, is with the Division of Endocrinology, Department of Medicine, UACOMP; Amanda J. Edmond, PA-C, is with the Department of Medical Oncology, Banner MD Anderson Cancer Center; Monica Camou, MSN, FNP-C, is with the Department of Medical Oncology, Banner MD Anderson Cancer Center; and Jiaxin Niu, MD, PhD, is with the Department of Medical Oncology, Banner MD Anderson Cancer Center.
Corresponding Author:
Jiaxin Niu, MD, PhD, Department of Medical Oncology, Banner MD Anderson Cancer Center, Gilbert, AZ 85234. email: Jiaxin.niu@bannerhealth.com
References
1. Li J, Zhang S, Zheng S, Zhang D, Qiu X. The BRAF V600E mutation predicts poor survival outcome in patients with papillary thyroid carcinoma: a meta-analysis. Int J Clin Exp Med. 2015;8(12):22246-22253.
2. Kim TH, Park YJ, Lim JA, et al. The association of the BRAF (V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118(7):1764-1773 doi:10.1002/cncr.26500
3. Aashiq M, Silverman DA, Na’ara S, et al. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel) 2019;11:1382.
4. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
5. Wirth LJ, Durante C, Topliss DJ, et al. Lenvatinib for the treatment of radioiodine-refractory differentiated thyroid cancer: treatment optimization for maximum clinical benefit. Oncologist. 2022;27(7):565-572. doi:10.1093/oncolo/oyac065
6. Gouda MA, Subbiah V. Expanding the benefit: dabrafenib/trametinib as tissue-agnostic therapy for BRAF V600E–positive adult and pediatric solid tumors. Am Soc Clin Oncol Educ Book. 2023;43:e404770. doi:10.1200/EDBK_404770
7. Busaidy NL, Konda B, Wei L, et al. Dabrafenib versus dabrafenib + trametinib in BRAF-mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial. Thyroid. 2022;32(10):1184-1192. doi:10.1089/thy.2022.0115
8. Thyroid cancer. National Comprehensive Cancer Network. 2024. https://www.nccn.org/patients/guidelines/content/PDF/thyroid-patient.pdf
9. Jaber T, Waguespack SG, Cabanillas ME, et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. 2018;103(10):3698-3705. doi:10.1210/jc.2018-00612
10. Leboulleux S, Benisvy D, Taieb D, et al. MERAIODE: a phase II redifferentiation trial with trametinib and 131I in metastatic radioactive iodine refractory RAS mutated differentiated thyroid cancer. Thyroid. 2023;33(9):1124-1129. doi:10.1089/thy.2023.0240
11. Iravani A, Solomon B, Pattison DA, et al. Mitogen-activated protein kinase pathway inhibition for redifferentiation of radioiodine refractory differentiated thyroid cancer: an evolving protocol. Thyroid. 2019;29(11):1634-1645. doi:10.1089/thy.2019.0143
11. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028-1035. doi:10.1158/1078-0432.CCR-14-2915
12. Toro-Tobon D, Morris JC, Hilger C, Peskey C, Durski JM, Ryder M. Clinical outcomes of radioactive iodine redifferentiation therapy in previously iodine-refractory differentiated thyroid cancers. Thyroid. 2024;34(1):70-81. doi:10.1089/thy.2023.0456
13. Jentzen W, Hoppenbrouwers J, van Leeuwen P, et al. Assessment of lesion response in the initial radioiodine treatment of differentiated thyroid cancer using 124I PET imaging. J Nucl Med. 2014;55(11):1759-1765. doi:10.2967/jnumed.114.144089
14. Schlumberger M, Challeton C, De Vathaire F, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37(4):598-605.

