- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
ASH 2024 Showcases Real-World Data Demonstrating Efficacy of Ruxolitinib Across Multiple Conditions
Two posters presented at the 2024 American Society of Hematology meeting bolster evidence supporting the long-term clinical benefits of ruxolitinib for polycythemia vera and chronic graft-vs-host disease.
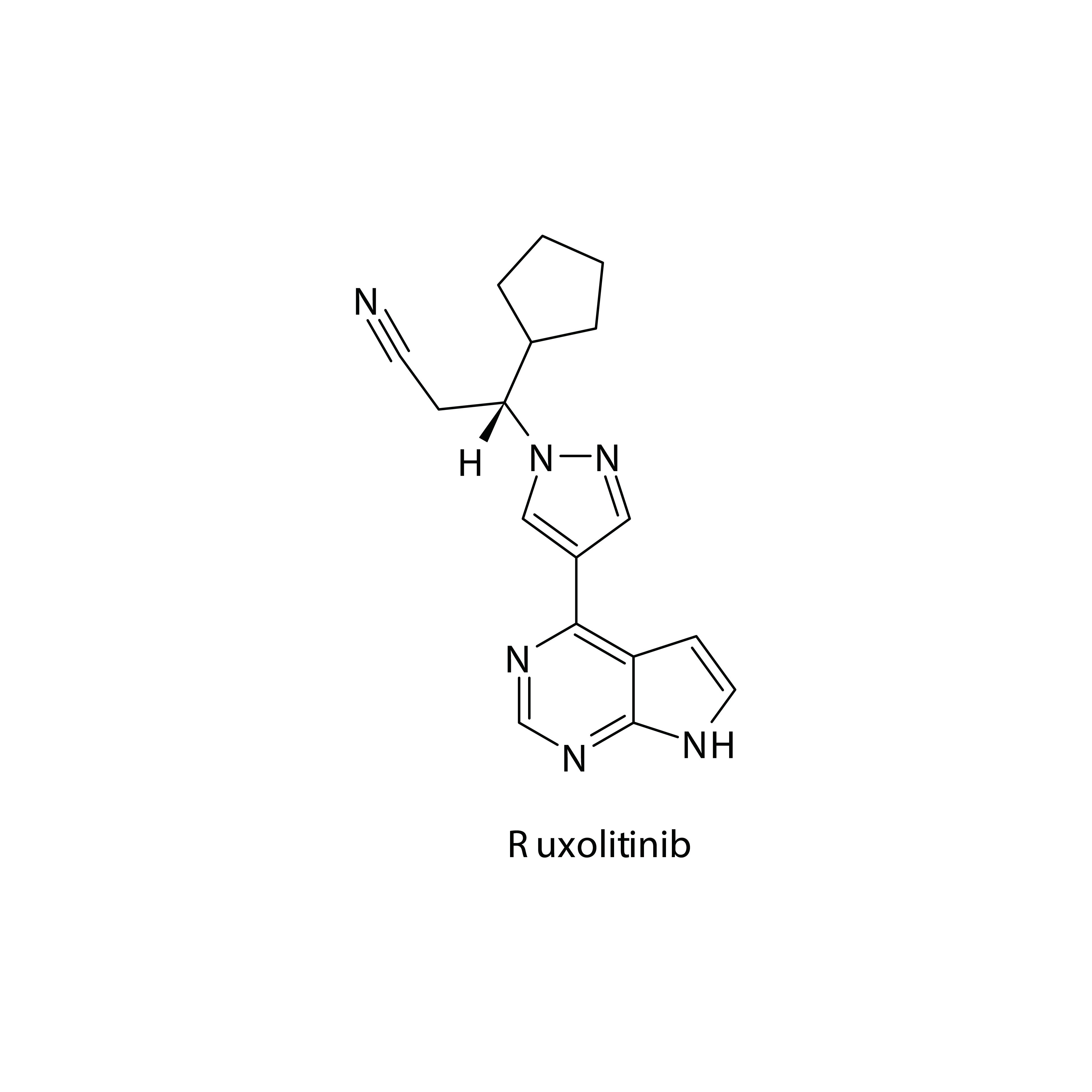
This year’s 2024 American Society of Hematology (ASH) Annual Meeting & Exposition featured novel research in the long-term safety and efficacy of ruxolitinib (Jakafi) for patients with polycythemia vera (PV) as well as those experiencing chronic graft-vs-host disease (cGVHD). Two poster presentations in particular shed light on the superiority of ruxolitinib over hydroxyurea (HU), and how ruxolitinib can allow patients to rely less on corticosteroids.
A retrospective study conducted by Pemmaraju and colleagues found that patients with PV exhibited improved hematocrit (Hct) levels and regulation of white blood cells (WBC) after switching from HU to the kinase inhibitor, ruxolitinib. At present, current national guidelines recommend HU—a cytoreductive treatment—or phlebotomies as the best practice for treating PV.
Ruxolitinib molecule | image credit: Basstock - stock.adobe.com
In a cohort of 443 patients with PV who switched to ruxolitinib, 10.6% (n = 47) were administered a combination therapy of HU (97.9%; n = 46) or pegylated-interferon (2.1%; n = 1) with ruxolitinib. Patients were aged nearly 69 years on average when they began HU, and their average time from diagnosis to beginning ruxolitinib was 31 months.
Out of 443 patients, 178 had sufficient Hct data for the analysis. A total of 41.6% (n = 74) registered with elevated Hct levels (at or above 45%) prior to their switch from HU to ruxolitinib. After beginning with ruxolitinib, these numbers dropped by the 3-, 6-, and 12-month marks to 22.5% (n = 40), 18% (n = 32), and 14.6% (n = 26), respectively.
There were 192 patients with sufficient WBC data, of whom 54.7% (n = 105) had elevated levels (at or above 11×109/L) after HU initiation prior to their switch. After ruxolitinib initiation, at the 3-, 6-, and 12-month marks, these numbers were 40.6% (n = 78), 46.4% (n = 89), and 45.8% (n = 88), respectively.
Platelet data was available for 200 patients and these levels were elevated (above 400) in 42.5% (n = 85) before their switch from HU to ruxolitinib; however, after switching, these numbers were 46% (n = 92), 46.5% (n = 93), and 42.5% (n = 85) at the 3-, 6-, and 12-month marks.
Considering these findings, the authors note how the “frequency of phlebotomy was numerically lower during ruxolitinib treatment vs during HU treatment. Taken together, these data suggest clinical benefits for patients switching to ruxolitinib following inadequate disease control with HU treatment.”
A complementary poster by Yu and colleagues showcased the potential of ruxolitinib to benefit patients with cGVHD, who were able to reduce their reliance on corticosteroids, as well as their steroid doses and the risk of associated complications. In this retrospective claims study, the authors expanded clinical knowledge regarding the impact ruxolitinib has on corticosteroid use in patients with cGVHD who have undergone allogeneic hematopoietic stem cell transplantation.
Claims data were gathered from health plan members belonging to Medicare, Medicare Advantage, and Medicaid. In total, 471 patients with cGVD were included who began ruxolitinib in between July 2019 and August 2022.
Those included were aged 46 years on average, with just over 15% being aged under 18 years, and all were followed for an average of 465 days after initiating ruxolitinib.
Following their cGVHD diagnosis, patients began ruxolitinib after 116 days on average. Dosing began at a median of 10 mg/day. Among adult patients, 58.4% underwent a dosage change at their refill, nearly 56% of which had dose increases and 44% had dose decreases. By the end of follow-up, just over 33% of patients had kept with their ruxolitinib treatment, which lasted a median of 389 days.
The median daily dose of corticosteroids was reduced from 40 mg to 12.5 mg after 180 days of ruxolitinib—a 69% reduction. The authors noted how patients who stared ruxolitinib within 30 days of their cGVHD diagnosis had a greater likelihood of tapering steroid use (72.3%) vs those who began between 30 and 100 days post diagnosis or after 100 days (59.3% and 58.5%, respectively; P = .03).
Additionally, the frequency of ambulatory visits was less following ruxolitinib initiation (68.4% vs 78.8%), as was the prevalence of inpatient stays (37.2% vs 56.1%) and visits to the emergency department (12.5% vs 15.3%), respectively.
The authors highlight that their results imply the significant and continual benefits of ruxolitinib in this patient population, both for mitigating complications related to steroid use and steroid dosage overall.
References
1. Pemmaraju N, Yu J, Vasudevan A, Qureshi H, Braunstein EM, Chojecki AL. Real-world treatment patterns and blood count control in patients with polycythemia vera who switched from hydroxyurea to ruxolitinib. Presented at: 66th ASH Annual Meeting & Exposition; December 7-10, 2024; San Diego, CA. Abstract 3813.
2. Moore KJ, Engel-Nitz NM, Ducharme M, Galvin J, Bhatt V, Lee CJ. Real-world ruxolitinib and corticosteroid treatment patterns in patients with chronic graft-versus-host disease in the United States. Presented at: 66th ASH Annual Meeting & Exposition; December 7-10, 2024; San Diego, CA. Abstract 4900.

