- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
340B Drug Pricing Program and Hospital Provision of Uncompensated Care
Participation in the 340B Drug Pricing Program by general acute care hospitals and critical access hospitals has not been associated with increased provision of uncompensated care.
ABSTRACT
Objectives: To evaluate whether hospital entry into the 340B Drug Pricing Program, which entitles eligible hospitals to discounts on drug purchases and intends for hospitals to use associated savings to devote more resources to the care of low-income populations, is associated with changes in hospital provision of uncompensated care.
Study Design: We analyzed secondary data on 340B participation and uncompensated care provision among general acute care hospitals and critical access hospitals from 2003 to 2015. We constructed an annual, hospital-level data set on hospital 340B participation from the Office of Pharmacy Information Systems and on uncompensated care provision from the Hospital Cost Reporting Information System.
Methods: Focusing on 2 periods of program expansion, we separately analyzed trends in uncompensated care costs for 340B-eligible general acute care hospitals and critical access hospitals, stratified by year of 340B program entry, including a stratum of eligible hospitals that never participated. We used a differences-in-differences approach to quantify whether there were differential changes in provision of uncompensated care after hospitals enter the 340B program relative to hospitals that did not participate or had not yet entered.
Results: We do not find evidence that hospitals increased provision of uncompensated care after entry into the 340B program differentially more than hospitals that never entered or had not yet entered the program.
Conclusions: Relying on hospitals to invest surplus into care for the underserved without marginal incentives to do so or strong oversight may not be an effective strategy to expand safety-net care.
Am J Manag Care. 2021;27(10):432-437. https://doi.org/10.37765/ajmc.2021.88761
Takeaway Points
Participation in the 340B Drug Pricing Program has not been associated with increases in hospital-reported uncompensated care provision, bringing into question whether the program is achieving its stated goal of freeing up resources that are devoted to the care of low-income populations.
- Our results add to a body of work that questions the extent to which program discounts are being used by general acute care and critical access hospitals to invest in care for low-income patients.
- Our findings suggest that relying on hospitals to invest surplus in care for the underserved without marginal incentives to do so or strong oversight is not a consistently effective strategy.
The 340B Drug Pricing Program is a federal program that entitles eligible hospitals to manufacturer discounts on purchases of drugs administered or prescribed in an outpatient setting. The discounted drugs can be provided to patients regardless of their ability to pay or their insurance coverage status. The 1992 statute under which the program was established states that program savings are intended to “stretch scarce federal resources as far as possible, reaching more eligible patients and providing more comprehensive services.” The program does not provide a direct incentive for hospitals to invest surplus in any specific way.
Although the program statute does not explicitly articulate how revenue from the 340B program should be used,1 federal agencies have since clarified that program resources should be used to better care for underserved patient populations.2 Uncompensated care, which includes charity care and other unreimbursed care provided to uninsured or underinsured patients, is one key mechanism by which hospitals devote resources to the care of vulnerable populations.3 Therefore, increases in hospital provision of uncompensated care following entry into the 340B program would be consistent with hospitals passing through discounts to patients in a manner intended by the program.
Program growth and evidence that 340B program incentives have increased hospital provision of drugs4,5 have led to increased scrutiny of whether 340B discounts benefit the underserved as intended. We examined trends in hospital-reported provision of uncompensated care during periods of 340B program expansion. We test whether hospital provision of uncompensated care increased following hospital entry into the 340B program for general acute care hospitals and critical access hospitals separately.
METHODS
We focused on 2 periods during which 340B participation by hospitals grew rapidly. The first period occurred after passage of the 2003 Medicare Modernization Act (MMA). The MMA expanded 340B eligibility to rural and small urban general acute care hospitals with sufficient shares of inpatient admissions for low-income patients as measured by the Disproportionate Share Hospital (DSH) adjustment percentage; following the MMA, participation increased among the newly eligible hospitals as well as previously eligible hospitals. (Although the reason for the increase in participation among already-eligible hospitals is unclear, it may be due to greater awareness about the program and organized industry efforts to support provider participation. For example, the first 340B Coalition Winter Conference—which aims to provide 340B entities with support and information on topics including program implementation, operations, compliance, contract pharmacy relationships, and inventory management, among other practical issues—was held in 2004.6) The second period followed the 2010 Affordable Care Act (ACA), which expanded eligibility to all critical access hospitals.5,7 We estimated changes in hospital-reported uncompensated care costs associated with program participation during these 2 periods of 340B growth for general acute care hospitals and critical access hospitals.
Study Data and Population
We used annual data from 2003 to 2015 from the Office of Pharmacy Affairs Information System,8 which identifies hospitals that participated in the 340B program by year, and the Hospital Cost Reporting Information System (HCRIS). Our study outcome was uncompensated care costs reported by hospitals in HCRIS, which is a measure of hospital costs associated with charity care, care provided to patients in means-tested government programs with low reimbursement rates (including Medicaid, the Children’s Health Insurance Program, and state and local indigent programs), and other unreimbursed care.
We analyzed 2 groups of hospitals. The first included general acute care hospitals in years 2003 to 2009, a period of rapid growth in participation among general acute care hospitals specifically; in fact, 78% of all general acute care hospitals in the 340B program in 2016 had entered by 2010. To be 340B eligible, general acute care hospitals were required to be nonprofit or publicly owned and had to have a DSH percentage exceeding 11.75% in the previous year. The DSH percentage is a function of several factors, including the proportion of a hospital’s admissions that are for Medicaid or low-income Medicare patients. We required general acute care hospitals to be 340B eligible in at least 1 year during the study period to be included in the main analysis, but results were similar when we controlled for eligibility or limited the analysis to hospitals eligible in every year.
Second, we examined critical access hospitals during years 2011 to 2015; all critical access hospitals became 340B eligible under the ACA in 2010. We excluded 2010 from the analysis because of inconsistent reporting resulting from a transition to a new HCRIS formula for calculating uncompensated care costs adopted by most hospitals in 2011.
We excluded the small number of hospitals that started and subsequently stopped 340B participation during the study period; our results were not substantively altered by their inclusion. To ensure at least 1 year of preparticipation data to estimate changes in uncompensated care costs from before to after program entry, we excluded general acute care hospitals participating before 2004 and critical access hospitals participating before 2012 from regression analyses.
We also excluded hospitals with missing uncompensated care data. Because reporting of uncompensated care was not mandatory during 2003 to 2009, 251 (18.7%) general acute care hospitals were excluded due to missing uncompensated care data. We did not find substantial differences in 340B participation, other hospital characteristics, or subsequent uncompensated care costs in 2011 to 2015 (when reporting was mandatory) between hospitals with missing and nonmissing data on uncompensated care costs in the 2003 to 2009 period (eAppendix Table 1 [eAppendix available at ajmc.com]). These findings suggest that missingness in the earlier period was unrelated to provision of uncompensated care and thus mitigate concerns that it was a source of bias in our analysis. For further details of the exclusion criteria and analysis of hospitals with and without missing outcomes data, see the eAppendix.
Statistical Analysis
We separately analyzed trends in uncompensated care costs for general acute care hospitals and critical access hospitals, stratified by year of 340B program entry, including a stratum of eligible hospitals that never participated. We used a differences-in-differences approach to quantify the extent to which hospitals differentially changed provision of uncompensated care after entering the 340B program relative to hospitals that did not participate or had not yet started participating. We estimated the following linear model:
Log(UCist) = β0 + β1340Bit + αi + αt + αs ∙ αt + eist
where Log(UCist) denotes the logged uncompensated care costs for hospital i in state s and year t; 340Bit is a time-varying annual indicator for hospital i’s participation status; αi denotes a vector of hospital fixed effects to control for time-invariant differences between hospitals; αt denotes a vector of year fixed effects; αs ∙ αt denotes a vector of fixed effects for every state-year combination in the data to control for state-specific time trends that might vary because of policy changes at the state level (eg, Medicaid expansion); and eist is a random error term. We log-transformed uncompensated care costs to estimate relative differences between cohorts. The coefficient for the time-varying 340B participation indicator approximated the quantity of interest: the percentage change in uncompensated care costs from before to after 340B participation for hospitals participating in a given year divided by the percentage change for hospitals not participating through that year, averaged over all years. In robustness tests, we specified the outcome in absolute dollars. We estimated robust standard errors clustered at the state level.9
In additional robustness tests, we weighted the regression by the number of hospital beds and specified the outcome as the log-transformed uncompensated care costs per hospital bed. One concern in analyses of general acute care hospitals is that our results could be biased by hospitals losing eligibility during the study period because our analysis requires hospitals to be eligible for 1 year during the study period at a minimum (this is not an issue in the analysis of critical access hospitals because all of these became 340B eligible under the ACA). To test whether potential changes in hospital eligibility status in subsequent years could be a source of endogeneity, we conducted 2 tests. First, we estimated the main model after limiting the analysis to hospitals that were eligible in every year of the study period starting in 2004. In a separate test, we controlled for whether a hospital was eligible in each year.
Analyses of Selection in 340B Participation and 340B Eligibility
An assumption necessary to interpret our results as causal effects of the 340B program is that time-varying, omitted variables do not affect changes in both participation status and levels of uncompensated care. We test for evidence on this assumption by comparing trends in the outcome between hospital cohorts during preparticipation years. Specifically, we tested for differences in uncompensated care cost trends during preparticipation years between entry-year cohorts of hospitals (including nonparticipants) by estimating the following model:
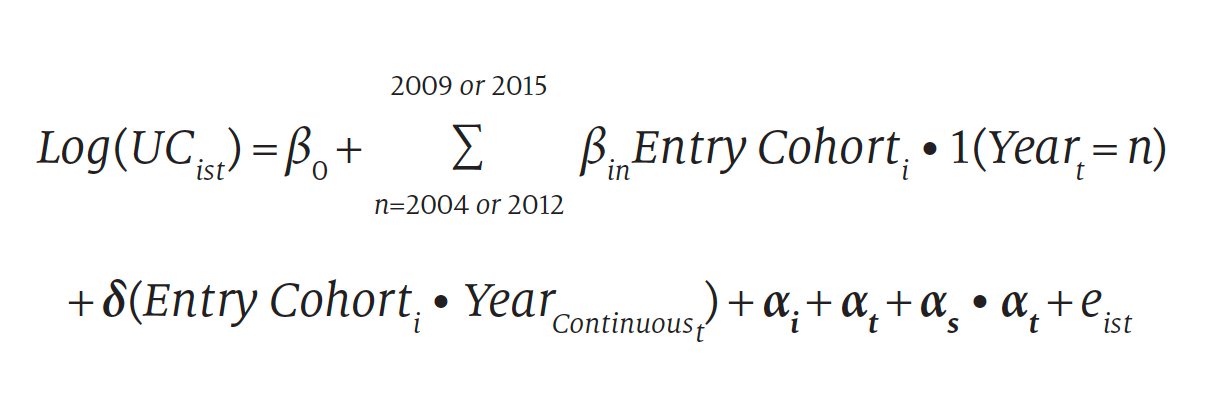
where Entry Cohorti ∙ YearContinuoust denotes an interaction term between a categorical variable assigned to each entry-year cohort including nonparticipants and a continuous year trend. Entry Cohorti ∙ 1(Yeart = n) is a vector of interaction terms between an indicator for hospital i’s 340B entry year and an indicator for each postentry year t, estimating a differential change in uncompensated care spending associated with each year of participation by entry-year cohort after adjusting for any preparticipation differences in trends. Because effects in postentry years will be absorbed by the vector of interactions Entry Cohorti ∙ 1(Yeart = n), the estimates δ describe the difference in the annual change in uncompensated care for each entry cohort during preentry years relative to the omitted cohort of hospitals that never participated. To assess differences in preentry trends between hospital cohorts, we calculate and report each cohort’s preentry trend from the coefficients in the vector δ and conduct a test of joint significance of the trend differences.
Another key assumption of our analysis, and prior work using a similar approach,10 is that changes in uncompensated care do not cause changes in participation by affecting hospital eligibility for the program. General acute care hospitals, for example, might increase the number of Medicaid patients served to increase their DSH payment percentage above the threshold for program eligibility, potentially resulting in an increase in uncompensated care spending associated with program entry. Because we defined our comparison groups based on observed changes in participation, random fluctuations or secular trends in uncompensated care that cause eligibility changes could also contribute to increases in uncompensated care spending that are associated with, but not caused by, program entry. This source of potential endogeneity bias is addressed in our sensitivity analysis, which restricts the analysis to hospitals eligible for the program in all years of the relevant expansion period, thereby eliminating changes in uncompensated care that caused changes in eligibility from estimates.
In prior work,11 we also tested extensively for evidence of hospital manipulation of DSH percentages to gain entry into the program and did not find evidence of this behavior. Specifically, we found minimal bunching of hospital DSH percentages just above the 11.75% DSH percentage threshold, at least in data through 2012. Emergence of such bunching in later years would not be consistent with strategic manipulation to enter the program because 78% of hospitals had already entered the program by 2010. We also found no evidence that general acute care hospitals with DSH percentages just above 11.75% in 2010 had exhibited greater increases in DSH percentages over preceding years or that characteristics of hospitals or their patients changed discontinuously at the threshold.
RESULTS
The study population consisted of 873 general acute care hospitals and 632 critical access hospitals. Figure 1 shows the percentage of all hospitals, general acute care hospitals, and critical access hospitals participating in the 340B program by year from 1996 to 2016, including the inflections in participation rates at the outset of the 2 expansion periods starting in 2004 and 2010. Following exclusions, among general acute care hospitals, the participation rate increased from 17% to 65% between 2004 and 2009. Among critical access hospitals in our study population, the participation rate increased from 45% to 79% between 2011 and 2015.
In their respective baseline years, general acute care hospitals reported mean uncompensated care costs of $26.1 million and critical access hospitals had mean uncompensated care costs of $1.6 million. (Note that these 2 measures are not directly comparable because of the change in the uncompensated care cost formula between the 2 study periods.) We do not find evidence that hospitals increased provision of uncompensated care in the years following entry into the 340B program differentially more than hospitals that never entered the program or had not yet entered the program. When stratified by year of 340B program entry, trends in log uncompensated care costs were similar across entry cohorts in nonparticipation years and did not systematically change following entry (Figure 2). On average, increases in uncompensated care costs from years before participation to years after program entry did not differ significantly from concurrent increases among hospitals not yet or never participating, both among general acute care hospitals (differential change, –4.6%; 95% CI, –9.7% to 0.7%; P = .09) and critical access hospitals (2.3%; 95% CI, –4.3% to 9.4%; P = .49) (Table). For comparison, mean annual changes in uncompensated care costs in nonparticipation years were 6% for general acute care hospitals and 3% for critical access hospitals. Hospitals that participated in the 340B program reported higher levels of uncompensated care compared with nonparticipating hospitals even prior to participation, demonstrating that cross-sectional differences between hospitals cannot be attributed to the 340B program.
Conclusions were similar when we specified the outcome as uncompensated care costs in absolute dollars (eAppendix Table 2). The differences-in-differences estimate of the increase in absolute uncompensated care costs associated with hospital 340B participation represents 3% of uncompensated care costs in the first year of the study period among general acute care hospitals (β = $791,400; P = .61) and 4% of uncompensated care costs in the first year of the study period for critical access hospitals (β = $66,200; P = .19). Analyses limiting the general acute care hospitals to those that were eligible in every year of the study period starting in 2004 and analyses using all hospitals from our main analysis but controlling for eligibility status in each year yielded results consistent with the main findings. Additional robustness tests, including controlling for changes in hospital 340B eligibility status and weighting by the number of beds, produced similar results (eAppendix Tables 3, 4, and 5).
Preparticipation trends differed minimally between hospital cohorts and were not systematically related to entry year or participation status, suggesting that 340B participation was not related to preexisting growth rates of uncompensated care and supporting our assumption that differences between cohorts would have remained constant in the absence of program participation (eAppendix Table 6). On average during nonparticipation years, trends in uncompensated care costs differed between cohorts of hospitals with different years of program entry by only 1.8% for acute care hospitals and by 0.6% for critical access hospitals. For 17 of 21 pairwise comparisons of acute care hospital cohorts and 10 of 10 pairwise comparisons of critical access hospital cohorts, trend differences were not statistically significant.
DISCUSSION
Our results indicate that participation in the 340B program has not been associated with increases in hospital-reported uncompensated care provision. Our analyses also highlight that cross-sectional differences between 340B and non-340B hospitals do not support inferences about the causal effects of the 340B program. We found that hospitals entering the 340B program earlier provided more uncompensated care, but these differences were present at baseline, before program participation and before program expansion, and did not widen between earlier and later entrants (or nonparticipants) after up to 6 more years of 340B discounts for the earlier entrants.
Our analyses add to evidence that questions whether the program is achieving its stated goal of freeing up resources to devote to the care of low-income populations. Our conclusions are consistent with prior work finding that the 340B program has not been associated with net increases in community benefit spending or outcomes for low-income populations.4,10 Our analysis extends this and other previous work by examining impacts of 340B entry on uncompensated care costs for both general acute care hospitals and critical access hospitals, the latter of which constitute a large proportion of new entrants following the ACA’s eligibility expansion.
A growing body of evidence that the program is not benefiting safety-net populations combined with evidence that it is contributing to hospital-physician consolidation and increases in hospital-based drug provision suggest the need for program reform. Reforms could include requiring that drugs purchased under the 340B program are provided to safety-net populations, establishing mechanisms for hospitals to pass on discounts to patients in the form of cost-sharing assistance, and increasing oversight and enforcement of how hospital surplus is used. More broadly, evidence to date on the program suggests that policies to finance safety-net care should more transparently and directly target resources to patient populations of interest, rather than indirectly by altering the profitability of some services, and include greater oversight and enforcement over how providers receiving resources use them. Recently, CMS proposed reducing Part B drug reimbursements for 340B hospitals to 22.5% less than average sales price.12 This reimbursement cut substantially reduces program funding for hospitals by reducing the profitability of administering 340B drugs, particularly for hospitals with a heavier Medicare payer mix. Instead, optimal policy might involve ending the 340B program and expanding or developing other programs to help low-income populations.
Limitations
Our study had several limitations. We could not assess the response of critical access hospitals entering the 340B program in the first 2 years of expanded eligibility because we lacked their preparticipation uncompensated care data. Moreover, hospital surplus from 340B discounts may have affected care for low-income patients in ways not reflected by the uncompensated care measure. In particular, we could not assess program effects on hospital closures. Next, our estimates would be biased if timing of hospital entry into the 340B program was associated with financial hardship and helped maintain uncompensated care provision that would have otherwise declined. However, such selection into participation would also be expected to be accompanied by differential trends in uncompensated care provision in the years prior to program entry, but we do not find evidence that preparticipation trends systematically differed between hospitals entering the program earlier vs later vs never. Because hospitals benefit from participation only if they administer discounted drugs, some eligible hospitals may not participate if they provide low volumes of outpatient drugs.13 Our analysis of preparticipation trends would suggest that hospital outpatient drug volume is not systematically related to trends in uncompensated care provision.
Relatedly, our research design does not control for confounding by time-varying predictors of uncompensated care that differentially changed after program entry. For example, if 340B participation was associated with displacement of subsidies from state and local governments, our estimates may underestimate the effects of participation. However, such displacement has not been documented to our knowledge, and such a regulatory mechanism at the hospital level would need to be relatively sophisticated because hospital eligibility can fluctuate over time and also seems unlikely given reports of limited program oversight.14 Finally, Medicare reimburses critical access hospitals for Part B (parenteral) drugs at 101% of costs, which limits savings accrued in the 340B program from administering Part B drugs to Medicare patients.15 Although these hospitals may still generate savings from the program via dispensing prescription drugs and administering parenteral drugs to commercially insured patients, the resulting surplus may be more limited than for acute care hospitals and insufficient to finance substantive increases in uncompensated care spending. Moreover, the high participation rate among critical access hospitals suggests that they benefit from program participation.
CONCLUSIONS
Our study sheds light on whether policies that direct unrestricted funds to hospitals for the purpose of expanding safety-net care in fact increase the provision of safety-net care. Prior research on whether hospitals cross-subsidize care for low-income populations is mixed and much of it predates our study period.16-18 Our findings suggest that relying on hospitals to invest surplus in care for the underserved without incentives to do so or strong oversight is not a consistently effective strategy. Policies that target resources directly to the intended populations (for example, through health insurance coverage expansions) may more reliably improve care and access compared with policies that encourage but do not require cross-subsidization by health care providers.
Author Affiliations: Department of Population Health, New York University School of Medicine (SMD), New York, NY; Department of Health Care Policy, Harvard Medical School (JMM), Boston, MA; Division of General Internal Medicine and Primary Care, Department of Medicine, Brigham and Women’s Hospital (JMM), Boston, MA.
Source of Funding: This work was supported by grants from the Agency for Healthcare Research and Quality (U19HS024072). Dr Desai was supported by grant number K01HS026980 from the Agency for Healthcare Research and Quality. The content was solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr Desai also thanks the Becker Friedman Institute Health Economics Fellowship for generous financial support. The funding sources did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Author Disclosures: Dr McWilliams reports having served as a consultant to Abt Associates Inc on an evaluation of the ACO Investment Model. Dr Desai reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (SMD, JMM); acquisition of data (SMD); analysis and interpretation of data (SMD, JMM); drafting of the manuscript (SMD); critical revision of the manuscript for important intellectual content (SMD, JMM); statistical analysis (SMD, JMM); and obtaining funding (SMD).
Address Correspondence to: Sunita M. Desai, PhD, Department of Population Health, New York University School of Medicine, 227 E 30th St #635, New York, NY 10016. Email: sunita.desai@nyu.edu.
REFERENCES
1. Public Health Service. Notice regarding section 602 of the Veterans Health Care Act of 1992 outpatient hospital facilities: final notice. Fed Regist. 1994;59(180):47884-47886.
2. Medicaid and CHIP Payment and Access Commission. Report to Congress on Medicaid and CHIP. Medicaid and CHIP Payment and Access Commission; 2016. Accessed January 1, 2021. https://www.macpac.gov/wp-content/uploads/2016/03/March-2016-Report-to-Congress-on-Medicaid-and-CHIP.pdf
3. Young GJ, Flaherty S, Zepeda ED, Singh SR, Cramer GR. Community benefit spending by tax-exempt hospitals changed little after ACA. Health Aff (Millwood). 2018;37(1):121-124. doi:10.1377/hlthaff.2017.1028
4. Desai S, McWilliams JM. Consequences of the 340B Drug Pricing Program. N Engl J Med. 2018;378(6):539-548. doi:10.1056/NEJMsa1706475
5. Jung J, Xu WY, Kalidindi Y. Impact of the 340B Drug Pricing Program on cancer care site and spending in Medicare. Health Serv Res. 2018;53(5):3528-3548. doi:10.1111/1475-6773.12823
6. Overview. 340B Coalition Winter Conference. Accessed January 7, 2021. https://www.340bwinterconference.org/wc21/Overview/Wc21/Overview.aspx?hkey=38eb8c31-d3c9-46b9-b2a3-68fa60a87cbb
7. Alpert A, Hsi H, Jacobson M. Evaluating the role of payment policy in driving vertical integration in the oncology market. Health Aff (Millwood). 2017;36(4):680-688. doi:10.1377/hlthaff.2016.0830
8. 340B Office of Pharmacy Affairs Information System. Health Resources & Services Administration. Accessed October 27, 2015. https://www.hrsa.gov/opa/340b-opais/index.html
9. Wooldridge JM. Econometric Analysis of Cross Section and Panel Data. 2nd ed. MIT Press; 2010.
10. Nikpay SS, Buntin MB, Conti RM. Relationship between initiation of 340B participation and hospital safety-net engagement. Health Serv Res. 2020;55(2):157-169. doi:10.1111/1475-6773.13278
11. Supplement to: Desai S, McWilliams JM. Consequences of the 340B Drug Pricing Program. N Engl J Med. 2018;378(6):539-548. doi:10.1056/NEJMsa1706475
12. CY 2021 Medicare hospital outpatient prospective payment system and ambulatory surgical center payment system proposed rule (CMS-1736-P). CMS. August 4, 2020. Accessed January 7, 2021. https://www.cms.gov/newsroom/fact-sheets/cy-2021-medicare-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center
13. Conti RM, Nikpay SS, Buntin MB. Revenues and profits from Medicare patients in hospitals
participating in the 340B Drug Discount Program, 2013-2016. JAMA Netw Open. 2019;2(10):e1914141. doi:10.1001/jamanetworkopen.2019.14141
14. 340B Drug Discount Program: increased oversight needed to ensure nongovernmental hospitals meet eligibility requirements. Government Accountability Office. December 2019. Accessed March 1, 2020. https://www.gao.gov/assets/710/703128.pdf
15. Medicare Learning Network. Critical access hospital. CMS. Accessed January 9, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/CritAccessHospfctsht.pdf
16. David G, Lindrooth RC, Helmchen LA, Burns LR. Do hospitals cross-subsidize? J Health Econ. 2014;37:198-218. doi:10.1016/j.jhealeco.2014.06.007
17. Baicker K, Staiger D. Fiscal shenanigans, targeted federal health care funds, and patient mortality. Q J Econ. 2005;120(1):345-386. doi:10.1162/0033553053327461
18. Norton EC, Staiger DO. How hospital ownership affects access to care for the uninsured. RAND J Econ. 1994;25(1):171-185.

Quality of Life: The Pending Outcome in Idiopathic Pulmonary Fibrosis
February 6th 2026Because evidence gaps in idiopathic pulmonary fibrosis research hinder demonstration of antifibrotic therapies’ impact on patient quality of life (QOL), integrating validated health-related QOL measures into trials is urgently needed.
Read More

