- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Two Steps Forward, One Step Back: 50 Years of Societal Value From LDL-C–Lowering Therapies
Low-density lipoprotein cholesterol (LDL-C)–lowering therapies have yielded significant value to society through reduced costs for both fatal and nonfatal cardiovascular disease events. The vast majority of this value has accrued to patients.
ABSTRACT
Objectives: To assess the evolving landscape of low-density lipoprotein cholesterol–lowering therapies (LLTs) and quantify their effect on cardiovascular disease (CVD)–related mortality and morbidity.
Study Design: Secondary data came from LLT clinical trials and 1999-2014 National Health and Nutrition Examination Survey (NHANES) data. 1996-2016 Medical Expenditure Panel Survey (MEPS) data were used to estimate LLT spending. Nonfatal CVD events prevented by LLTs were calculated from clinical trials and NHANES. The value of nonfatal events prevented was calculated as the product of event treatment costs and the number of events prevented. The value of mortality reduction was calculated as the product of a value of a life-year and the life expectancy gain from LLTs. This was compared with LLT spending estimated using MEPS.
Methods: Total LLT expenditures were calculated based on MEPS LLT utilization and expenditure data. Values of prevented hospitalizations, prevented CVD events, and other LLT utilization–related outcomes were pulled from the published literature.
Results: Combined, statins and ezetimibe prevented 2.8 million nonfatal heart attacks and 1.7 million nonfatal strokes from 1999 to 2014. Statin use generated $2.6 trillion in societal value through CVD deaths avoided from 1987 to 2014, and 85% accrued to patients.
Conclusions: LLTs have yielded significant societal value, and the majority of this value has accrued to patients.
Am J Manag Care. 2021;27(4):162-168. https://doi.org/10.37765/ajmc.2021.88618
Takeaway Points
Low-density lipoprotein cholesterol–lowering therapies (LLTs) have yielded significant value to society through reduced costs for both fatal and nonfatal cardiovascular disease (CVD) events. The vast majority of this value has accrued to patients.
- This study contributes to the literature on the value of preventive medicine and to the growing body of evidence that LLTs generate significant economic and clinical value to both patients and society as a whole.
- We demonstrate that statins and ezetimibe have yielded significant value to society through the reduction of costs associated with both fatal and nonfatal CVD events.
- It is important to understand the value generated by these therapies, as this information can inform the development of treatment guidelines and managed care policy.
Despite 70 years of progress due to advances in both prevention and treatment,1 cardiovascular disease (CVD) remains the leading cause of death and the largest contributor to medical costs in the United States.2 In 2017, approximately 95 million adults (aged ≥ 20 years) in the United States had high cholesterol (≥ 200 mg/dL).3 Elevated low-density lipoprotein cholesterol (LDL-C) in particular remains a key target for therapy because it is associated with an increased risk of CVD-related events such as nonfatal myocardial infarction (MI), nonfatal stroke, coronary revascularization, and CVD mortality.4 In 2013-2014, an estimated 30% of adults (aged ≥ 20 years) in the United States had high LDL-C (≥ 130 mg/dL).5,6 Of particular concern, 63% of patients with previous MI exhibited LDL-C levels above the goal of 70 mg/dL despite being on some form of LDL-C–lowering therapy (LLT).7-11
In response to continuing unmet need, the LLT landscape has evolved significantly over the past half-century. Statins, which became available in the United States in 1987, are among the world’s best studied drugs.12,13 There is unequivocal evidence on the value of statins in primary and secondary CVD prevention. Based on large outcomes trials and meta-analyses, statins lower CVD event risk by 22% per 38.7 mg/dL (1 mmol/L) reduction in LDL-C.8,14,15 The West of Scotland Coronary Prevention Study (WOSCOPS) demonstrated a durable survival benefit for long-term (≥ 5 years) statin users.16
Additionally, 2 other classes of drugs have yielded positive results in combination with statins by reducing LDL-C: ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.8-11 However, evidence on their clinical value remains mixed. For PCSK9 inhibitors, the ODYSSEY Outcomes trial demonstrated a survival benefit in the secondary prevention setting,17 but the FOURIER trial (evolocumab) and subsequent trial meta-analyses did not.11,18 Although ezetimibe demonstrated a benefit in the composite end point of CV death/myocardial infarction (MI)/hospitalization for unstable angina/coronary revascularization beyond 30 days/stroke, it did not demonstrate a significant protective effect for CV mortality alone.19 However, some have argued that this is due to the relatively low mean baseline LDL-C levels of trial participants (95 mg/dL in both arms of IMPROVE-IT).19,20
Prior literature has established that statins alone have produced value far in excess of their costs. The initial introduction and uptake of statins in 1987 prompted the publication of several cost-effectiveness studies using traditional cost-benefit analysis.21-23 More recently, Grabowski et al expanded on traditional cost-benefit analysis to approximate the societal value of survival gains generated by statin use24 and found that statins generate significant value to society, the majority of which accrues to patients. There is also some evidence of societal value from PCSK9 inhibitor drugs. Jena et al estimated that reducing LDL-C by 50% among patients with a high risk of experiencing a CV event who have insufficient response to other LLTs could prevent up to 14.2 million major adverse coronary events (MACEs), including 1.6 million CVD deaths, by 2035.25
Given CVD’s high disease burden and the evolution of LLT innovation and clinical guidelines, it is informative to update the prior literature by incorporating more recent information on statins and other LLTs.
We build upon and broaden the seminal Grabowski et al study on statins by (1) updating the estimates through 2014, which is the most recently available year of observed data; (2) estimating nonfatal events and hospitalizations avoided; and (3) incorporating other LLTs. To gain further understanding of the relationship between LLT use and reductions in CVD mortality and morbidity, this study utilized a quantitative analysis to calculate the societal benefits and costs of LLTs to patients initiating LLT for primary and secondary prevention from 1987, when statins were introduced, through 2014.
METHODS
Study Design Overview
Descriptive analyses of the rates of LLT use and LLT spending over time were conducted using both National Health and Nutrition Examination Survey (NHANES) (1999-2014) and Medical Expenditure Panel Survey (MEPS) (1996-2016) data, and the associations between LDL-C levels and CV mortality and hospitalizations were identified in the literature. The basis for the Grabowski methodology, which we build upon, is the notion that social value estimates ought to begin with real-world data on statin users. NHANES is the only nationally representative health survey with objective data on lipid levels, and MEPS is the only nationally representative database on health care expenditures. These databases were used to estimate the number of statin users (for < 5 years and ≥ 5 years), the number of ezetimibe users, nonfatal MIs and strokes among LLT users, annual expenditures on statins, and annual expenditures on ezetimibe. As with most large-scale surveys, the NHANES and MEPS data have some gaps. For this reason, we relied on the literature to derive estimates of the relative risk of CVD events for statin users vs nonusers. Our approach, however, does not capture reductions in quality of life from drug adverse effects or improvements in quality of life from improved health. In focusing exclusively on survival and costs, we follow the bulk of the existing literature on social value.26-28 The rationale for this strategy in the literature is that survival gains are more straightforward to monetize than quality-of-life harms and benefits, and they are likely to be more quantitatively meaningful in this context.
The total number of nonfatal CVD events prevented over time was calculated using HRs from published clinical trials and NHANES data on the number of LLT users and frequency of self-reported nonfatal CVD events among these users. Events avoided were calculated as the difference between real-world CVD events observed among LLT users and events that would have occurred in the absence of LLT use. The latter was calculated by taking the real-world CVD events among LLT users in NHANES and deducting the difference in events between treated and untreated patients in LLT trials. This yields an estimate of total nonfatal CVD events prevented. We then multiply by prior estimates of nonfatal CVD event costs3,29 to construct the value of prevention. The value of mortality reductionwas calculated as the product of the value of a statistical life-year (VSLY; $150,000) and the life expectancy gain from the WOSCOPS clinical trials for statins (for primary prevention), respectively.16 The life expectancy gains for those using statins for at least 5 years estimated from WOSCOPS equaled 0.145 years per user. The life expectancy gains for those using statins for 4 or fewer years was estimated at 0.03 years based on the WOSCOPS trial data, as in Grabowski et al.24 We do not include survival benefits for PCSK9 inhibitors because the trial evidence is mixed on PCSK9 inhibitor survival benefits.19,20 Further details are illustrated in Figure 1 and described below.
A targeted literature review was conducted to identify studies on the relationship between LLT use and (1) CVD morbidity (eg, heart attack, stroke), (2) CVD event hospitalizations, and (3) CVD mortality. The goals of the literature review were to provide context for the study, identify relevant parameters for quantifying the reductions in CVD mortality and hospitalizations attributable to LLTs through reductions in LDL-C, and inform comparisons of the relative degree of innovativeness of a particular LLT or category of LLTs. Search strategies were implemented using Google Scholar, PubMed, and the Cochrane Library. Reviewers screened the titles and abstracts to identify publications that appeared to capture the relevant information in accordance with the selection criteria.
Statistical Analysis
Descriptive analyses of the rates of LLT utilization and LDL-C levels, as well as all other patient characteristics (age, race, blood pressure readings, weight, height, smoking status, physical activity, prescription drug use, and relevant medical conditions), were conducted using data from all continuous NHANES survey cycles (eg, 1999-2000 to 2013-2014). The unadjusted number and percentage of Americans 18 years and older using LLTs by LLT type and American College of Cardiology/American Heart Association statin benefit group and across study years were calculated. The number of users from 2015 to 2030 was projected based on the linear time trend estimated from observed number of statin users in 1999-2014. The Guideline on the Assessment of CVD Risk30 was used to estimate individuals’ 10-year atherosclerotic CVD risk, where 10-year risk of 7.5% or greater is considered elevated risk.30 We conducted 2 sensitivity analyses: (1) We explored the effect of halving the estimated growth of statin use from 2015 to 2030, and (2) we explored the use of predicted values for statin utilization and statin costs from nonlinear regressions and generated CIs around these estimates. The sensitivity analysis methods are described in the eAppendix materials (eAppendix available at ajmc.com).
Mean annual expenditures on LLTs were calculated based on LLT utilization and expenditures from MEPS. All expenditure outcomes were inflated to 2018 Q1 US$ using the all-urban US Bureau of Labor Statistics Consumer Price Index.31 All statistics were estimated using the appropriate survey weights and variance primary sampling unit to account for the complex survey design and to produce nationally representative estimates.
Information on cardiac revascularizations is not available in NHANES; thus, we could not estimate the number of revascularizations prevented among LLT users. Nonfatal events prevented (EP) by year and LLT type (ie, statins and ezetimibe) were calculated as the difference between the number of CVD events we would expect to see in LLT users if they did not use LLTs (E1) and the number of events we see in LLT users when they do use LLTs (E2) (ie, EP = E1 – E2). We estimated E1 based on the relative risk of CVD events in clinical trials for statins and ezetimibe16,19 (HRCT) and the observed frequency of CVD events (ie, MI or stroke) among LLT users in NHANES in the previous 12 months, Pr(E2). Events prevented were calculated as:
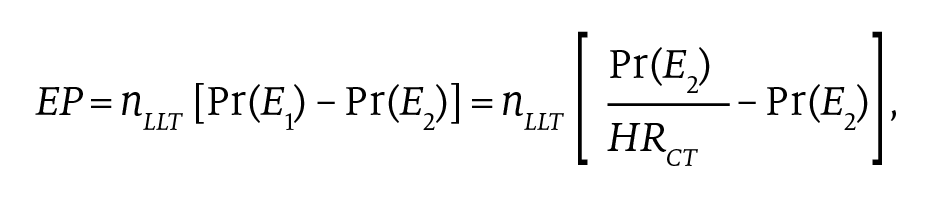
where nLLT denotes the number of individuals using an LLT in a given year. Utilization of LLTs and frequency of nonfatal MIs and strokes among LLT users by LLT type were estimated in the NHANES data.
The expected life-years gained from LLT use (ie, reduction in CVD mortality) were estimated from the hazard/survival curves reported in the key LLT clinical trial WOSCOPS (statins).16 The survival curve coordinates were extracted for both trial arms and used to estimate the life expectancy for each curve. Life expectancy was estimated as the integral of the survival curve. Mean life expectancy gain was calculated as the difference between the trial treatment and control arms.
The value of prevented hospitalizations for nonfatal MI and nonfatal stroke was calculated using the estimated number of nonfatal events prevented by LLT and mean costs for these events from the literature.30-41 Mortality reductions were valued based on widely used estimates of the VSLY ($150,000)42-44 and the expected life-years gained from LLT use that were estimated from the hazard/survival curves reported in WOSCOPS for statins.16,17 Health gains were discounted at 3% per year. Annual social value was calculated as:
Vt = 150,000 × 0.145 × exp(−0.03[t−2018]) × nt5+ + 150,000 × 0.03 × exp(−0.03[t−2018]) × nt<5,
where nt5+ is the incidence of 5-year statin use and nt<5 is the prevalence of 0 to 4 years of statin use.
RESULTS
LLT Costs, LDL-C Levels, CVD Events, and Life-Years Saved
Mean annual expenditures on LLTs hovered around $900 per treated patient per year from 1999 to 2008, then fell sharply after several statin patent expirations in the mid- to late 2000s. In 2016, annual expenditures on LLTs averaged $517 per treated patient.
Mean LDL-C levels among LLT users fell sharply between 1999 and 2006, from approximately 120 mg/dL to 100 mg/dL, and remained at approximately 100 mg/dL through 2014. Mean LDL-C levels among LLT candidates (ie, those in a statin benefit group not using an LLT) had mean LDL-C level of approximately 130 mg/dL from 1999 to 2014.
Using the NHANES data, we estimated that statins and ezetimibe prevented a combined 2.8 million nonfatal heart attacks and 1.7 million nonfatal strokes from 1999 to 2014. Note that we predicted zero events prevented by ezetimibe for some years because no nonfatal strokes or MIs were observed among the small number of ezetimibe users in those years.
Social Value
In the United States alone, we estimate that statin use generated $2.6 trillion (2018 US$) in cumulative social value in prevented CVD deaths from 1987 to 2014 and that patients retained 85% ($2.2 trillion of $2.6 trillion) of this value. Assuming a VSLY of $150,000, the cost to society of each life-year gained by statin users equals $18,500 (Figure 2 and Figure 3). Even at a more modest VSLY of $50,000, statin use generated $869 billion (2018 US$) in cumulative social value in prevented CVD deaths from 1987 to 2014, and patients retained $463 billion of this value. From 2015 to 2030, the analysis predicts that statin use will generate an additional $1.5 trillion (2018 US$) in cumulative social value in prevented CVD deaths and that patients will retain 93% ($1.4 trillion of $1.5 trillion) of this value. The societal value of these innovations is greatest late in the product life cycle, as the number of treated patients and the duration of treatment increases and as patents expire.
The first sensitivity analysis, where we reduced the predicted growth in statin utilization from 2015 to 2030 by half, resulted in a modest decrease in the aggregate benefits of statin use, from $4.1 trillion to $3.7 trillion over the period 1987 to 2030. In this scenario, the vast majority (87%) of the benefits still accrue to patients, and the cost per life-year gained remains well under conventional methods that typically use a threshold of $50,000 for high-value services. In the second sensitivity analysis, designed to produce CIs, the cumulative benefits and costs from 1987 to 2030 are both lower, at $3.2 trillion (95% CI, $2.9 trillion–$3.5 trillion) and $299 billion (95% CI, $269 billion–$330 billion), respectively. However, the proportion accruing to patients is a bit higher, 93% vs 87%, in the baseline analysis (see eAppendix Figure).
In terms of morbidity, statins and ezetimibe have prevented a combined 2.8 million nonfatal heart attacks between 1999 and 2014, which would have cost society $258 billion to treat. Statins and ezetimibe have prevented a combined 1.7 million nonfatal strokes over the same period, which would have cost society $37 billion to treat (eAppendix Table, Figure 4, and Figure 5).
In sum, about 88% ($2.16 trillion) of total LLT value was accrued as mortality reduction from 1999 to 2014, and about 12% was accrued through reductions in morbidity.
DISCUSSION
Over time, new LLTs are developed and brought to market, and long-term outcomes are studied in the real-world setting. As the LLT landscape evolves, so too do treatment guidelines. Given the immense clinical and economic burden of CVD, it is informative and meaningful to better understand the societal value generated by LLTs.
This study contributes to the literature on the value of preventive medicine45,46 and to a growing body of evidence that LLTs generate significant clinical and economic value to society.24,25,47 The cost to society of each life-year gained by statin users was $18,500, which would be considered cost-effective by conventional methods that typically use a threshold of $50,000 for high-value services48 and commonly used thresholds of $100,000 to $150,000 per quality-adjusted life-year.49 We demonstrate that statins and ezetimibe have yielded significant value to society through the reduction of costs associated with both fatal and nonfatal CVD events. In the United States alone, statin use generated $2.6 trillion in cumulative social value in prevented CVD deaths from 1987 to 2014, and patients retained 85% of this value. Expressed in 2008 US$, our estimates of the social value of statin use for 1987 to 2008 are very similar to those of Grabowski et al: $1.29 trillion vs $1.25 trillion. In a similar study, Jena et al estimated that PCSK9 inhibitor use among high-risk individuals with inadequate response to other LLTs in the United States could prevent an additional 14.2 million MACEs, including 1.6 million CVD deaths, between 2015 and 2035 and generate $2.9 trillion in cumulative value.25
Between 1999 and 2014, statins and ezetimibe prevented a combined 2.8 million nonfatal heart attacks, which would have cost society $258 billion to treat. Over the same period, statins and ezetimibe prevented a combined 1.7 million nonfatal strokes, which would have cost society $37 billion to treat. These findings are consistent with a recent study by Cutler et al, which found that use of medications for hypertension, high cholesterol (ie, LLTs), and diabetes contributed significantly to the slowing of Medicare expenditure growth between 1999 and 2012.47
Many LLTs have been investigated over the previous 40 years, yet only a few have gone on to generate value to patients and the returns to innovators that finance the large number of unsuccessful LLT trials. The societal value of these innovations is greatest late in product life cycle, after patent expiry.
Limitations
This study has some limitations. First, we do not observe LDL-C, LLT use, and expenditures on LLTs for the same individuals, and we therefore use data from multiple data sources (ie, NHANES and MEPS).
Second, to the extent that the frequency of nonfatal stroke and MI among LLT users, and ezetimibe users in particular, is underestimated in the NHANES data, the estimated number and value of nonfatal events prevented by LLTs use are understated. Also, as discussed earlier, we did not attribute a mortality benefit to ezetimibe use. Therefore, our estimated benefits of ezetimibe use are understated to the extent that there is a mortality benefit from ezetimibe use in the real world (eg, in patients with LDL-C > 100 mg/dL).
Third, our estimates do not include any benefits from increases in health-related quality of life attributable to LLTs or costs from decreases in health-related quality of life attributable to adverse effects. Fourth, our methodological framework does not adequately capture considerations from a cost perspective that may be incurred by patients upon initiating novel LLTs. Fifth, as discussed above, we did not attribute a mortality benefit to PCSK9 inhibitor use. Thus, our estimated benefits of LLT use are understated in regard to the mortality benefit gained from PCSK9 inhibitor use in the real world (eg, among patients who achieve LDL-C < 70 mg/dL with the addition of a PCSK9 inhibitor).
Finally, as with other social value studies, it is difficult to rigorously quantify every source of uncertainty and its implications for the variability of our estimates. For example, real-world clinical benefits may depart from clinical trial benefits. Utilization projections introduce uncertainty above and beyond the variability in the underlying data sets. For these reasons, we follow prior literature by presenting mean estimated effects and transparently documenting our approach to constructing them. Also following prior studies, we avoid presenting CIs in the main analyses because it is difficult to capture in full all the potential sources of uncertainty possible.24 However, for completeness, we construct them in a sensitivity analysis, which yields qualitatively similar conclusions and appears in the eAppendix material.
CONCLUSIONS
Meaningful social value has accrued from innovation in the LLT landscape through the reduction of costs associated with both fatal and nonfatal CVD events. Most of this value was generated by statins, ezetimibe, and PCSK9 inhibitors.
Author Affiliations: PRECISIONheor (JPM, LMZ, KE, DNL), Los Angeles, CA; Merck & Co (LL, DL, FS), Kenilworth, NJ; Weill Cornell Medical College (LL), New York, NY.
Source of Funding: Merck & Co.
Author Disclosures: Dr MacEwan and Ms Zhao are employed by PRECISIONheor, a research consulting firm owned by Precision for Medicine and compensated by Merck to conduct the study. Ms Everson is employed by Precision Xtract. Drs Liu and Lautsch are employees of and stock owners in Merck & Co. Dr Sun is a former employee of Merck. Dr Lakdawalla is a consultant to Precision Health Economics and holds equity in Precision Medicine Group.
Authorship Information: Concept and design (JPM, LL, DL, FS, DNL); acquisition of data (JPM, KE); analysis and interpretation of data (JPM, KE, LL, DL, DNL); drafting of the manuscript (JPM, LMZ); critical revision of the manuscript for important intellectual content (JPM, LMZ, LL, DL, DNL); statistical analysis (JPM); provision of patients or study materials (JPM); obtaining funding (JPM, LL); administrative, technical, or logistic support (JPM, LMZ, LL, FS); supervision (JPM, FS, DNL); and programming (KE).
Address Correspondence to: Joanna P. MacEwan, PhD, PRECISIONheor, 11100 Santa Monica Blvd, Ste 500, Los Angeles, CA 90025. Email: Joanna.MacEwan@precisionvh.com.
REFERENCES
1. Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120(2):366-380. doi:10.1161/CIRCRESAHA.116.309115
2. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia; Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933-944. doi:10.1161/CIR.0b013e31820a55f5
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;136(10):e146-e603. doi:10.1161/CIR.0000000000000485
4. Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303(6797):276-282. doi:10.1136/bmj.303.6797.276
5. Benjamin EJ, Muntner P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
7. Barkas F, Elisaf M, Rizos EC, Liberopoulos E. Bridging the treatment gap in patients at ‘extreme’ cardiovascular risk: evidence from a lipid clinic. Atherosclerosis. 2019;281:216-218. doi:10.1016/j.atherosclerosis.2018.11.022
8. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
9. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
12. Kandutsch AA, Packie RM. Comparison of the effects of some C27-, C21-, and C19-steroids upon hepatic sterol synthesis and hydroxymethylglutaryl–CoA reductase activity. Arch Biochem Biophys. 1970;140(1):122-130. doi:10.1016/0003-9861(70)90016-0
13. Hoffmann U, Lu MT, Olalere D, et al; REPRIEVE investigators. Rationale and design of the Mechanistic Substudy of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J. 2019;212:1-12. doi:10.1016/j.ahj.2019.02.011
14. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407-415. doi:10.1016/S0140-6736(18)31942-1
15. Mihaylova B, Emberson J, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. doi:10.1016/S0140-6736(12)60367-5
16. Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM; West of Scotland Coronary Prevention Study Group. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357(15):1477-1486. doi:10.1056/NEJMoa065994
17. ODYSSEY Outcomes: results suggest use of PCSK9 inhibitor reduces CV events, LDL-C in ACS patients. American College of Cardiology. March 10, 2018. Accessed February 21, 2019. https://www.acc.org/latest-in-cardiology/articles/2018/03/05/15/53/sat-9am-odyssey-outcomes-cv-outcomes-with-alirocumab-after-acs-acc-2018
18. Giugliano RP, Pedersen TR, Park JG, et al; FOURIER Investigators. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390(10106):1962-1971. doi:10.1016/S0140-6736(17)32290-0
19. Eisen A. IMProved Reduction of Outcomes: Vytorin Efficacy International Trial—IMPROVE-IT. American College of Cardiology. Updated February 5, 2018. Accessed October 8, 2018. https://www.acc.org/latest-in-cardiology/clinical-trials/2014/11/18/16/25/improve-it
20. Navarese EP, Andreotti F, Raggi P, et al. Baseline low-density lipoprotein cholesterol to predict the extent of cardiovascular benefit from lipid-lowering therapies: a review. Eur Heart J Cardiovasc Pharmacother. 2018;5(1):47-54. doi:10.1093/ehjcvp/pvy038
21. Johannesson M, Jönsson B, Kjekshus J, Olsson AG, Pedersen TR, Wedel H. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. N Engl J Med. 1997;336(5):332-336. doi:10.1056/NEJM199701303360503
22. Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R; Heart Protection Study Collaborative Group. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20,536 individuals. Lancet. 2005;365(9473):1779-1785. doi:10.1016/S0140-6736(05)63014-0
23. Goldman L, Weinstein MC, Goldman PA, Williams LW. Cost-effectiveness of HMG-CoA reductase inhibition for primary and secondary prevention of coronary heart disease. JAMA. 1991;265(9):1145-1151. doi:10.1001/jama.1991.03460090093039
24. Grabowski DC, Lakdawalla DN, Goldman DP, et al. The large social value resulting from use of statins warrants steps to improve adherence and broaden treatment. Health Aff (Millwood). 2012;31(10):2276-2285. doi:10.1377/hlthaff.2011.1120
25. Jena AB, Blumenthal DM, Stevens W, Chou JW, Ton TGN, Goldman DP. Value of improved lipid control in patients at high risk for adverse cardiac events. Am J Manag Care. 2016;22(6):e199-e207.
26. Yin W, Penrod JR, Maclean R, Lakdawalla DN, Philipson T. Value of survival gains in chronic myeloid leukemia. Am J Manag Care. 2012;18(suppl 11):S257-S264.
27. Philipson TJ, Jena AB. Who benefits from new medical technologies? estimates of consumer and producer surpluses for HIV/AIDS drugs. Forum Health Econ Policy. 2006;9(2). doi:10.2202/1558-9544.1005
28. Philipson T, Eber M, Lakdawalla DN, Corral M, Conti R, Goldman DP. An analysis of whether higher health care spending in the United States versus Europe is ‘worth it’ in the case of cancer. Health Aff (Millwood). 2012;31(4):667-675. doi:10.1377/hlthaff.2011.1298
29. O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29(8):693-704. doi:10.2165/11584620-000000000-00000
30. Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63(25, pt A):2886. doi:10.1016/j.jacc.2014.02.606
31. Consumer Price Index. US Bureau of Labor Statistics. Accessed May 20, 2018. https://www.bls.gov/cpi/
32. Alirocumab for treatment of high cholesterol: effectiveness and value.Institute for Clinical and Economic Review. February 15, 2019. Accessed March 2, 2021. https://icer.org/wp-content/uploads/2020/10/ICER_Alirocumab_Final_NEU_021519.pdf
33. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
34. Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med. 2016;374(20):1954-1964. doi:10.1056/NEJMra1406944.
35. Heart disease facts. CDC. Updated September 8, 2020. Accessed September 21, 2018. https://www.cdc.gov/heartdisease/facts.htm
36. Population, total for United States. Federal Reserve Bank of St. Louis. Updated July 2, 2020. Accessed December 17, 2018. https://fred.stlouisfed.org/series/POPTOTUSA647NWDB
37. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. Accessed December 17, 2018. https://hcupnet.ahrq.gov/#setup
38. Wang G, Zhang Z, Ayala C, Dunet DO, Fang J, George MG. Costs of hospitalization for stroke patients aged 18-64 years in the United States. J Stroke Cerebrovasc Dis. 2014;23(5):861-868. doi:10.1016/j.jstrokecerebrovasdis.2013.07.017
39. Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001-2008. JAMA. 2011;305(17):1769-1776. doi:10.1001/jama.2011.551
40. Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, et al. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation. 2016;133(10):967-978. doi:10.1161/CIRCULATIONAHA.115.019904
41. Person-years of life lost. National Cancer Institute. Accessed December 17, 2018. https://web.archive.org/web/20110701094442/http:/progressreport.cancer.gov/doc_detail.asp?pid=1&did=2007&chid=76&coid=730&mid=#estimate
42. Aldy JE, Viscusi WK. Age differences in the value of statistical life: revealed preference evidence. Rev Environ Econ Policy. 2007;1(2):241-260.
43. Aldy JE, Viscusi WK. Adjusting the value of a statistical life for age and cohort effects. Rev Econ Stat. 2008;90(3):573-581.
44. Viscusi WK, Aldy JE. The value of a statistical life: a critical review of market estimates throughout the world. J Risk Uncertain. 2003;27:5-76. doi:10.1023/A:1025598106257
45. Maciosek MV, Coffield AB, Flottemesch TJ, Edwards NM, Solberg LI. Greater use of preventive services in U.S. health care could save lives at little or no cost. Health Aff (Millwood). 2010;29(9):1656-1660. doi:10.1377/hlthaff.2008.0701
46. Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118(5):576-585. doi:10.1161/CIRCULATIONAHA.108.190186
47. Cutler DM, Ghosh K, Messer KL, Raghunathan TE, Stewart ST, Rosen AB. Explaining the slowdown in medical spending growth among the elderly, 1999-2012. Health Aff (Millwood). 2019;38(2):222-229. doi:10.1377/hlthaff.2018.05372
48. Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332-342. doi:10.1177/0272989X0002000310
49. Overview of the ICER value assessment framework and update for 2017-2019. Institute for Clinical and Economic Review. Accessed March 2, 2021. https://icer.org/wp-content/uploads/2020/10/ICER-value-assessment-framework-Updated-050818.pdf

Quality of Life: The Pending Outcome in Idiopathic Pulmonary Fibrosis
February 6th 2026Because evidence gaps in idiopathic pulmonary fibrosis research hinder demonstration of antifibrotic therapies’ impact on patient quality of life (QOL), integrating validated health-related QOL measures into trials is urgently needed.
Read More

