- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
This Week in Managed Care: June 13, 2015
This week the American Diabetes Association Scientific Sessions was held in Boston with results on 2 cardiovascular outcomes trials for diabetes drugs, and FDA advisory panels voted in favor of 2 PCSK9 inhibitors.
The American Diabetes Association Scientific Sessions featured results from 2 important cardiovascular outcomes trials for diabetes drugs: ELIXA for lixisenatide and TECOS for sitagliptin. To see the full coverage from the annual meeting, visit our conference page.
FDA advisory panel recommendations were released for 2 PCSK9 inhibitors. One panel voted 11-4 to recommend approval for Amgen’s evolocumab, but not without a lengthy discussion of which patients would be eligible to receive the drug. The other panel voted 13-3 to approve for the long-term treatment of adult patients with primary hypercholesterolemia or mixed dyslipidemia alirocumab.
The Government Accountability Office's latest report on 2011 Medicaid claims data found that Medicaid paid $9.6 million in claims filed regarding services for 200 people who had died and detailed providers who are now excluded from federal health propgrams.
Finally, the newest issue of The American Journal of Accountable Care is now available online and includes a recap of the ACO and Emerging Healthcare Delivery Coaltion spring live meeting. Learn more about the Coalition and how to join.
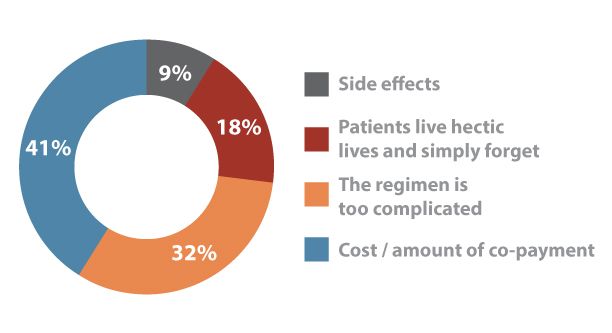
Poor adherence to medication has been a major topic at the 75th Scientific Sessions of the American Diabetes Association. Which item is most likely to cause patients to fail to take medication as prescribed?