- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Ruxolitinib Cream Shows Long-Term AD Control in Children Aged 2 to 11 Years
New data highlight efficacy, safety, and time off treatment with as-needed ruxolitinib cream in pediatric atopic dermatitis.
At the Society for Pediatric Dermatology Annual Meeting, 2 poster presentations from the TRuE-AD3 (NCT04921969) study demonstrated that children aged 2 to 11 years with mild to moderate atopic dermatitis (AD) achieved long-term disease control and meaningful time off treatment using as-needed ruxolitinib cream. Both strengths of the topical Janus kinase inhibitor were well tolerated over 52 weeks, even among those with more extensive disease at baseline.
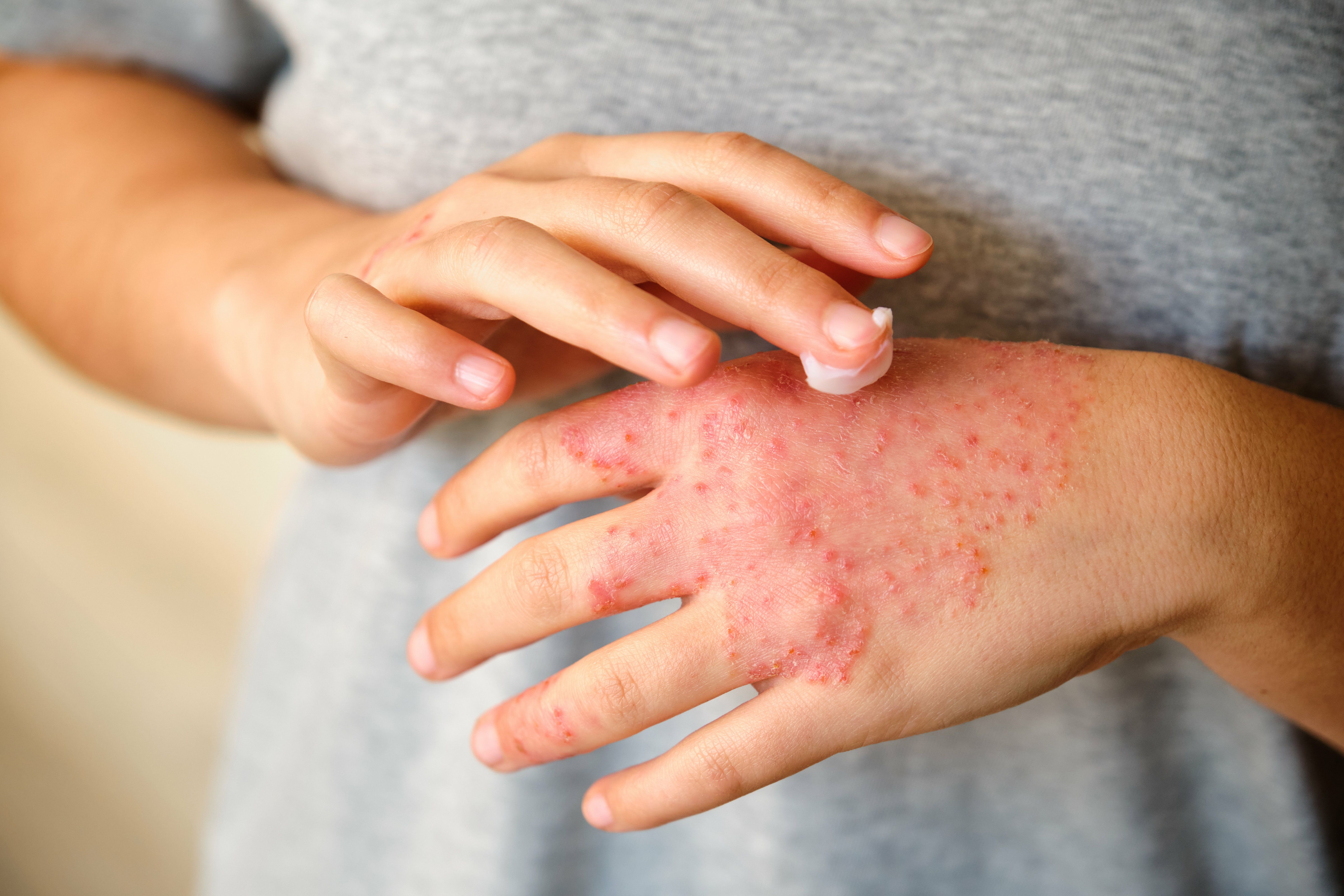
The first poster from the TRuE-AD3 study focused on evaluating long-term disease control and safety of ruxolitinib cream in children aged 2 to 11 with moderate and/or more extensive AD, defined as an Investigator’s Global Assessment (IGA) score of 3 and/or 10% or greater affected body surface area (BSA) at baseline.1 In this randomized, double-blind trial, children received twice-daily ruxolitinib cream (0.75% or 1.5%) or vehicle for 8 weeks, followed by 44 weeks of as-needed treatment with either ruxolitinib cream strength.
Among 180 children initially randomized to ruxolitinib with an IGA score of 3, 71.4% to 74.6% achieved clear or almost clear skin (IGA 0/1) at week 52, and mean affected BSA decreased to 2.0% to 2.3%. Similar improvements were observed in the subset with 10% or greater BSA at baseline. Both concentrations of ruxolitinib cream were well tolerated, with no serious treatment-related adverse events reported, supporting its sustained efficacy and safety in managing moderate pediatric AD.
The second poster evaluated long-term disease control and time off treatment among children aged 2 to 11 with mild to moderate AD who received ruxolitinib cream in the TRuE-AD3 study.2 After an initial 8-week randomized treatment period, patients who had received ruxolitinib cream (0.75% or 1.5%) continued into a 44-week long-term safety (LTS) period with as-needed application. Among those who applied at least 1 dose during the LTS period (n = 119 for 0.75%; n = 114 for 1.5%), affected BSA decreased from 9.9% to 11.1% at baseline to 1.9% to 2.0% at week 52.
Clear or almost clear skin (IGA 0/1) was achieved at least once during the LTS period by 80.7% (ruxolitinib, 0.75%) and 91.2% (ruxolitinib, 1.5%) of patients, and these scores were maintained for 60.0% and 69.0% of visits, respectively. Additionally, median days off treatment due to lesion clearance totaled 136.0 and 151.0 for the 0.75% and 1.5% groups, respectively, accounting for approximately half the LTS period. Both regimens were well tolerated with no serious treatment-related adverse events, underscoring the effectiveness of as-needed ruxolitinib cream in maintaining disease control and reducing treatment burden in pediatric AD.
Together, these findings from the TRuE-AD3 study reinforce the long-term efficacy, safety, and practical benefits of ruxolitinib cream as an as-needed monotherapy for children with mild to moderate AD. Whether managing more extensive disease or maintaining remission over time, ruxolitinib cream enabled substantial lesion clearance, prolonged periods off treatment, and a favorable safety profile over 52 weeks. These results offer promising evidence to support its use as a long-term treatment option tailored to the needs of pediatric patients and their caregivers.
References
1. Eichenfield LF, Simpson EL, Armstrong AW, et al. 52-week ruxolitinib cream disease control and safety in children with moderate/more extensive atopic dermatitis. Poster presented at: Society for Pediatric Dermatology Annual Meeting 2025; July 23-26, 2025; Seattle, WA. Abstract POS-05.
2. Eichenfield LF, Joyce JC, Lee LW, et al. Ruxolitinib cream demonstrated long-term disease control with time off treatment in children with atopic dermatitis. Poster presented at: Society for Pediatric Dermatology Annual Meeting 2025; July 23-26, 2025; Seattle, WA. POS-18.
The Importance of Examining and Preventing Atrial Fibrillation
August 29th 2023At this year’s American Society for Preventive Cardiology Congress on CVD Prevention, Emelia J. Benjamin, MD, ScM, delivered the Honorary Fellow Award Lecture, “The Imperative to Focus on the Prevention of Atrial Fibrillation,” as the recipient of this year’s Honorary Fellow of the American Society for Preventive Cardiology award.
Listen
Promoting Equity in Public Health: Policy, Investment, and Community Engagement Solutions
June 28th 2022On this episode of Managed Care Cast, we speak with Georges C. Benjamin, MD, executive director of the American Public Health Association, on the core takeaways of his keynote session at AHIP 2022 on public health policy and other solutions to promote equitable health and well-being.
Listen
