- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Across 8 Trials, Ruxolitinib Cream Shows Favorable Safety in Pediatric AD
A poster at the Society for Pediatric Dermatology Annual Meeting presented an integrated safety analysis showing rare or no serious adverse events linked to topical Janus kinase inhibitor use in children with atopic dermatitis (AD).
At the Society for Pediatric Dermatology (SPD) 50th Annual Meeting, new research synthesizing data from 8 clinical trials provided reassuring evidence on the long-term safety of ruxolitinib cream in children and adolescents with atopic dermatitis (AD). The analysis examined adverse events associated with Janus kinase (JAK) inhibitors, particularly those flagged in systemic use, and found that serious safety concerns were rare or absent in pediatric patients using the topical formulation.
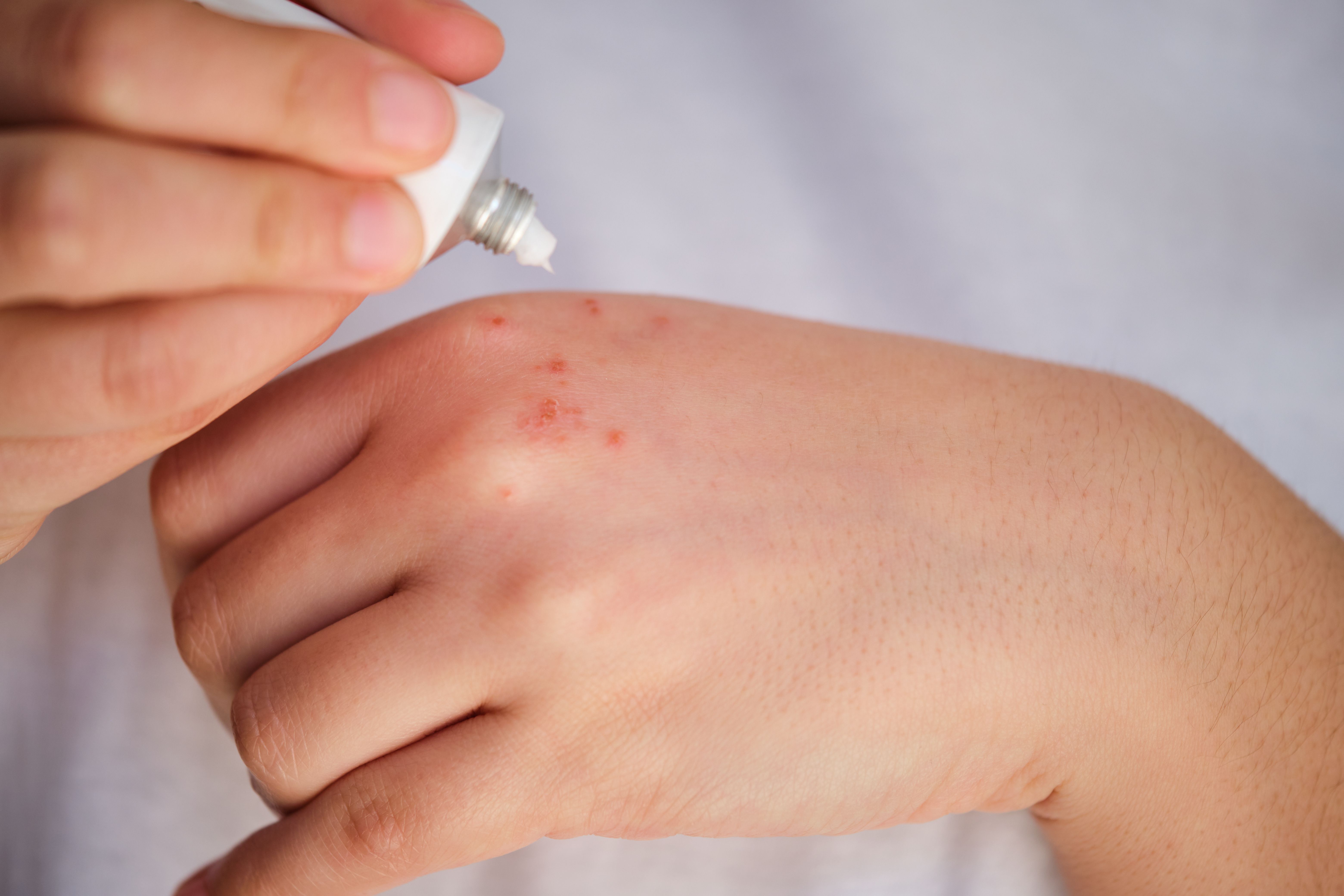
Ruxolitinib cream, a selective JAK1/JAK2 inhibitor formulated for topical use, has proven effective in clinical trials for managing mild to moderate AD by targeting inflammation at the site of disease activity. While more research is needed to confirm efficacy in certain conditions, topical JAK inhibitors are emerging as a lower-risk option for managing diseases such as psoriasis, AD, and vitiligo, with fewer concerns about systemic adverse effects.2
Systemic JAK inhibitors have raised safety concerns in chronic inflammatory conditions such as rheumatoid arthritis, prompting classwide warnings for both oral and topical formulations.1 These include risks of serious infections, major adverse cardiovascular events (MACE), thromboembolism, malignancy, and death. These risks have created an urgent need to evaluate whether similar risks apply to topical ruxolitinib, especially in pediatric populations.
To address this, researchers conducted an integrated safety analysis across 8 clinical trials of ruxolitinib cream involving children and adolescents aged 2 to 17 years. These included 4 phase 3 studies, 1 phase 2 trial, 2 maximum-use studies, and 1 safety/pharmacokinetics study. In total, 767 patients contributed to 509.62 patient-years (PY) of ruxolitinib cream exposure, with 306 patients using the 0.75% strength (204.64 PY) and 461 using the 1.5% strength (304.98 PY).
No MACE, thromboembolic events, malignancies, or fatalities were reported across any of the studies, and only 2 patients experienced serious infections, both while using the 1.5% strength. Neither event was deemed related to the study drug by investigators. Additionally, there were no serious infections in the 0.75% group. Application site reactions were rare, and plasma concentrations of ruxolitinib remained low, consistent with the topical route of administration.
The data revealed that systemic exposure to ruxolitinib was far below the threshold associated with JAK-mediated myelosuppression, even in patients enrolled in maximum-use studies who applied cream to up to 92% of their body surface area. Occasional plasma concentration outliers were observed but had no clinical consequences. These findings support the mechanism of topical delivery—targeting localized inflammation while minimizing systemic absorption.
The exposure-adjusted incidence rates were calculated for each AE of interest, reinforcing the conclusion that topical ruxolitinib does not carry the same risk profile as oral JAK inhibitors. These findings are particularly important given the vulnerability of pediatric patients and the growing use of JAK inhibitors in dermatology.
References
1. Siri DD, Lee LW, Stein Gold LF, et al. Ruxolitinib cream in pediatric patients with atopic dermatitis: Integrated safety analysis of 8 clinical trials. Poster presented at: Society for Pediatric Dermatology Annual Meeting 2025; July 23-26, 2025; Seattle, WA. Abstract POS-19.
2. Santoro C. Topical ruxolitinib emerges as promising therapy for diverse inflammatory skin conditions. AJMC®. June 17, 2025. Accessed July 24, 2025. https://www.ajmc.com/view/topical-ruxolitinib-emerges-as-promising-therapy-for-diverse-inflammatory-skin-conditions
The Importance of Examining and Preventing Atrial Fibrillation
August 29th 2023At this year’s American Society for Preventive Cardiology Congress on CVD Prevention, Emelia J. Benjamin, MD, ScM, delivered the Honorary Fellow Award Lecture, “The Imperative to Focus on the Prevention of Atrial Fibrillation,” as the recipient of this year’s Honorary Fellow of the American Society for Preventive Cardiology award.
Listen
Promoting Equity in Public Health: Policy, Investment, and Community Engagement Solutions
June 28th 2022On this episode of Managed Care Cast, we speak with Georges C. Benjamin, MD, executive director of the American Public Health Association, on the core takeaways of his keynote session at AHIP 2022 on public health policy and other solutions to promote equitable health and well-being.
Listen
