- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Noninvasive Ventilation at Home Reduces Mortality in COPD With CRF
This study explores the association between receiving noninvasive ventilation at home and mortality, hospitalizations, and emergency department visits in patients with chronic obstructive pulmonary disease (COPD) with chronic respiratory failure (CRF).
ABSTRACT
Objectives: Patients with chronic respiratory failure resulting from chronic obstructive pulmonary disease (COPD-CRF) have limited treatment options and poor health outcomes. We examined the effect of noninvasive ventilation at home (NIVH) treatment on all-cause mortality, hospitalizations, and emergency department (ED) visits.
Study Design: Retrospective cohort study.
Methods: Using Medicare claims data between 2012 and 2017, we divided patients with COPD-CRF into a treatment group, defined by NIVH receipt within 2 months of CRF diagnosis, and a control group without NIVH receipt in the entire follow-up period. We modeled time to death, first hospitalization, and first ED visit. Cox regressions were performed, mitigating selection bias using stabilized inverse probability of treatment weights with regression controls. Sensitivity analyses with time-varying exposure to NIVH were conducted on the full sample irrespective of treatment timing.
Results: We identified 410 patients treated with NIVH and 36,247 controls. We observed a reduced risk of hospitalizations (HR, 0.790; 95% CI, 0.592-0.988), ED visits (HR, 0.571; 95% CI, 0.457-0.686), and mortality (HR, 0.617; 95% CI, 0.462-0.772). The benefit of NIVH diminished over time for mortality and ED visits but remained constant for hospitalizations. However, no survival benefit was observed in the sensitivity analyses that accounted for immortal-time bias; further exploration suggests that earlier NIVH treatment following CRF diagnosis may be an important factor in improving survival outcomes.
Conclusions: Patients with COPD-CRF who received NIVH had statistically significant reductions in hospitalizations and ED visits compared with patients not treated with NIVH. Further research is needed to examine the effect of NIVH on mortality.
Am J Manag Care. 2021;27(9):e308-e315. https://doi.org/10.37765/ajmc.2021.88743
Takeaway Points
This study explores the association between receiving noninvasive ventilation at home (NIVH) and mortality, hospitalizations, and emergency department (ED) visits in patients with chronic obstructive pulmonary disease (COPD) with chronic respiratory failure (CRF).
- Patients with COPD and CRF who were prescribed NIVH had statistically significant reductions in risk of death (38.3%), hospitalizations (21.0%), and ED visits (42.9%) compared with similar patients not treated with NIVH.
- NIVH is associated with a 1-year risk difference of 13.0% for mortality, 8.4% for first hospitalization, and 17.6% for first ED visit, yielding relative risk reductions of 30.7%, 12.9%, and 20.1%, respectively. The numbers needed to treat are 7.7 to prevent a death, 12.0 to prevent a first hospitalization, and 5.7 to prevent a first ED visit.
Chronic obstructive pulmonary disease (COPD) affects approximately 7% of the US population.1 COPD is the third-leading cause of death, at 120,000 deaths per year,2 and accounts for 1.5 million emergency department (ED) visits and 700,000 hospitalizations annually. COPD produces approximately $50 billion in annual direct and indirect costs to society.3-5
As COPD progresses from mild to severe disease associated with chronic respiratory failure (CRF), it becomes deadlier and consumes more health care resources. In an international cohort of patients with CRF due to COPD (COPD-CRF), the 3-year mortality rate was almost 20%.6 CRF is debilitating to older patients, who have a higher mortality rate than their younger counterparts.6 CRF negatively affects quality of life (QOL) and limits a patient’s ability to engage in the activities of daily living.7
Because COPD has no cure, treatment is palliative; goals include reducing mortality and hospitalizations and improving QOL. Many treatment strategies, including noninvasive ventilation at home (NIVH), have been employed to mitigate adverse outcomes, with mean adherence having been reported at 6 hours of use per day.8 Despite conflicting evidence of efficacy, NIVH use among patients with COPD-CRF is increasing. The number of patients prescribed NIVH in the United States grew from 120 in 2009 to 27,921 in 2015.9,10 However, NIVH still remains a rarely used intervention, with less than 5% of US patients with COPD-CRF receiving this therapy.9
Early studies, summarized in a 2013 Cochrane review, found no benefit for NIVH use in patients with COPD-CRF.10 However, recent studies have reported significant improvements in QOL and reductions in hospitalization rates.11-16 Additionally, a European randomized controlled trial showed a significant mortality reduction in hypercapnic patients with COPD when treated with high-intensity NIVH.13 The findings of this trial have been replicated in other notable trials across Europe, Australia, and China, lending more evidence to support the efficacy of NIVH in reducing mortality and hospital readmissions.17 These studies were included in a recent meta-analysis reporting improvements in clinical outcomes associated with NIVH use.18
Despite these reports, evidence for the effectiveness of NIVH for patients with COPD-CRF in the United States is limited. To address this gap, we conducted a retrospective study on the effectiveness of NIVH in the Medicare COPD-CRF population.
METHODS
Data Source
A patient-level analytic file was created using the Medicare Limited Data Set (LDS) between 2012 and 2017. The LDS contains information on claims through Part A and Part B for a randomly selected 5% sample of the complete Medicare fee-for-service population.19
Study Cohort
We restricted our sample to patients with a CRF diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 518.53, 518.83, 518.84; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes J95.822, J96.10, J96.11, J96.12, J96.20, J96.21, J96.22) and a concurrent COPD diagnosis (ICD-9-CM codes 490, 491.0, 491.1, 491.8, 492.0, 492.8, 491.20, 491.21, 491.22, 496; ICD-10-CM codes J40, J41.1, J41.8, J42, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, J44.9). All patients had at least 18 months of continuous enrollment in Medicare, except those who died in that period. Additionally, patients were excluded if they resided outside the United States at any point during the enrollment period, were ever enrolled in Medicare Advantage, had dementia in the 6-month preindex period (ICD-9-CM codes 290.x, 294.1, 331.2; ICD-10-CM codes F00.x-F03.x, F05.1, G30.x, G31.1), or ever had obstructive sleep apnea (OSA) (ICD-9-CM code 327.23; ICD-10-CM code G47.33). Patients with dementia were excluded because of the difficulty of such patients tolerating NIVH.20 Because NIVH is an effective treatment for OSA, we excluded patients with OSA to ensure that any benefit observed due to NIVH treatment was due to its effect on COPD-CRF.21 Figure 1 shows the attrition table.
We divided the final sample into 2 groups: (1) a treatment group consisting of patients who received NIVH (pre-2016 Healthcare Common Procedure Coding System [HCPCS] codes E0460, E0461, E0464; 2016 and 2017 HCPCS code E0466) within 2 months of CRF diagnosis, and (2) a control group consisting of patients who did not receive NIVH at any point in the follow-up period. The index date was the date of CRF diagnosis.
Outcomes
The primary study outcome was all-cause mortality (time to death). The secondary outcomes were time to first hospital admission and time to first ED visit after index date. All patients were followed until event occurrence or were censored if patients did not experience the event by the end of the study period (December 31, 2017).
Statistical Analysis
Time-to-event analysis.The associations between NIVH and outcomes were estimated using time-to-event analyses (Cox regressions). The semiparametric Cox model assumes that the ratio of the treatment group hazard to the control group hazard is constant over time. Violation of this assumption may yield biased estimates.22 We found that the proportional hazard assumption was violated by regressing Schoenfeld residuals on a time index.23 As such, we included a time-by-treatment interaction (treatment*log[time]) in the regressions.24 This approach results in the simultaneous estimation of 2 parameters related to the treatment effect: an HR describing the difference in event rates at the start of the analysis period and a second parameter describing the rate of change in the HR over time.
Due to the observational nature of this study, 2 methods were employed to control for confounding and selection bias: inverse probability of treatment weighting (IPTW; described later) and inclusion of covariates as regression controls. Using both methods is a conservative approach, as either can provide consistent estimates of treatment effects if properly specified.25
The demographics controlled for and used for matching included race/ethnicity, gender, age, and region of the country. We also used the Charlson Comorbidity Index (to control for the presence of chronic diseases), total health care spending 6 months pre–index date, county-level smoking prevalence, and comorbid hypercapnia.
Propensity score weighting. We estimated the probability of receiving NIVH (the propensity score) as a function of observable demographic and clinical covariates. We then reweighted the sample using stabilized IPTW weights as described by Austin.26 This approach reweights the treatment and control groups such that differences in observable characteristics between the 2 groups are minimized. Additionally, it permits the estimation of an average treatment effect across the entire population of eligible patients. The IPTW and propensity score weighting approach has been used in a number of recent cardiovascular retrospective studies and has been shown effective at reducing bias compared with other methods.27-29
Bootstrapping to calculate statistical significance. Because both the propensity score estimation procedure and the regression models were computed with error, we used bootstrapping to calculate standard errors (SEs) that incorporate both sources of uncertainty. Austin and Small have previously demonstrated that this approach correctly estimates SEs in 2-part models.30 We generated 500 bootstrap samples.
Models. We estimated HRs for all-cause mortality, first hospitalization, and first ED visit using 4 different models: (1) a naive comparison of the unweighted treatment and control groups, (2) model 1 adding demographic and clinical covariates, (3) comparison of the IPTW sample including demographic and clinical covariates, and (4) model 3 with bootstrapped SEs.31 Model 4 is the preferred model because it incorporates both techniques to address confounding and uses SEs that address joint uncertainty.
Risk difference, relative risk reduction, and numbers needed to treat. In addition to reporting the HRs for each end point, we implemented the methodology of Austin to estimate the percentage of patients who experience an event in the treatment group (PT) and control group (PC), the risk difference (RD), relative risk reduction (RRR), and numbers needed to treat (NNT) at 1 year after index date using our preferred model (Cox model with demographic and clinical covariates, and bootstrapped SEs on the IPTW sample).32 The RD, RRR, and NNT were calculated as:
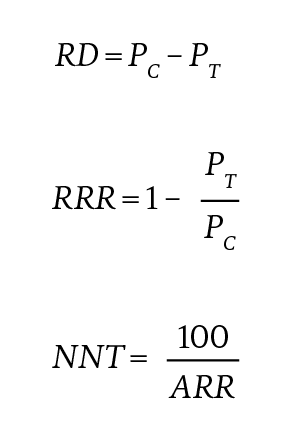
with ARR indicating absolute risk reduction.
Sensitivity analysis. We incorporated the shortest possible window of receiving treatment after index that provided a treatment group large enough for our main analysis to have sufficient study power to reduce the possibility of immortal-time bias.33 However, this study design does not completely eliminate the concern of immortal-time bias. As such, we implemented a Cox model with time-varying exposure to NIVH for each end point as sensitivity analyses. We estimate 3 models: (1) a naive Cox model, (2) a Cox model with demographic and clinical covariates, and
(3) model 2 with bootstrapped standard errors. In this time-varying covariate approach, individuals switch between the treatment and control groups depending on whether they are on or off treatment, and thus propensity score matching would not be appropriate. We reconstructed the analytic file to the patient–treatment period level and included all patients with COPD-CRF into the study sample irrespective of timing of NIVH receipt. The results are reported in the eAppendix (available at ajmc.com).
RESULTS
Descriptive Statistics
Our sample consisted of 36,657 total patients: 410 patients with COPD-CRF treated with NIVH within 2 months of CRF diagnosis (treatment group) and 36,247 controls. Table 1 summarizes the demographic and clinical characteristics of both groups before and after IPTW implementation. Before reweighting, statistically significant differences in baseline demographic characteristics and clinical factors existed between the treatment and control group. Notably, the treatment group was younger than the control group and had substantially lower 6-month pre–index date total health care spending. IPTW achieved good balance between the treatment and control groups: No statistically significant differences remained, except for gender (P < .05).
Primary End Point Results
Table 2 describes results for the time-to-event analysis for each end point and the 4 analysis specifications. Model 1 is the naive comparison, model 2 is regression with controls using the original sample, model 3 is regression with controls using the reweighted population using IPTW, and model 4, the preferred model, is model 3 with bootstrapped SEs. For all-cause mortality and ED visits, regression of Schoenfeld residuals indicated a violation of the proportional hazard assumption (results not shown). As such, a time-by-treatment interaction (treatment*log[time]) was included for all outcomes in all model specifications.
NIVH was associated with improved survival in patients with COPD-CRF. Each model specification revealed a statistically significant reduction in risk of death associated with NIVH at the start of the analysis period. In model 4, patients receiving NIVH had a 38.3% reduction in risk of death compared with those not receiving NIVH (HR, 0.617; 95% CI, 0.462-0.772) at index date. The associated decay parameter (1.001) describes the extent to which this benefit is time varying; in other words, the decreased risk of death for those receiving NIVH diminishes by 0.1% per day (HR, 1.001; 95% CI, 1.001-1.001). The 2 parameters can be combined to estimate the HR for NIVH at time t. For example, a year after diagnosis, the HR is estimated to be 0.617 × (1.001)365 = 0.889. As such, the survival benefit of NIVH disappeared after 69 weeks. The time-varying nature of the survival benefit of NIVH is evident in Figure 2, which plots the survival rate across a 1-year time frame estimated using the preferred model (model 4). The survival benefit is largest in the period immediately following diagnosis and then diminishes over time.
Table 3 presents results for the year after COPD-CRF diagnosis. One year from index date, 29.4% of patients receiving NIVH had died and 42.4% of controls had died. This translates to an RD of 13.0% and an RRR of 30.7% for all-cause mortality. The NNT to prevent a death at 1 year was 7.7.
Secondary End Point Results
NIVH was associated with improvement in time to first hospitalization and to first ED visit (Table 2). In our preferred model (model 4), receiving NIVH resulted in a 21.0% reduction in risk of hospitalization (HR, 0.790; 95% CI, 0.592-0.988) at index date and a 42.9% reduction in risk of ED visit (HR, 0.571; 95% CI, 0.457-0.686) at index date. The benefit conferred by NIVH in reducing the risk of time to first ED visit declined by 0.1% per day (HR, 1.001; 95% CI, 1.000-1.001), whereas the reduced risk of first hospitalization remained constant (Table 2). The HR of NIVH for ED visits was 0.571 × (1.001)365 = 0.822 after a year. There was no decreased risk of an ED visit for patients receiving NIVH after 81 weeks.
Table 3 shows that after 1 year, 56.3% of patients prescribed NIVH had been hospitalized compared with 64.6% of controls. This translates to an RD of 8.4% and an RRR of 12.9% for first hospitalization. The NNT to prevent a first hospitalization at 1 year was 12.0. Additionally, after 1 year, 70.0% of patients prescribed NIVH had been to the ED compared with 87.7% of controls. This translates to an RD of 17.6% and an RRR of 20.1% for ED visits. The NNT to prevent a first ED visit at 1 year was 5.7.
DISCUSSION
We estimated the comparative effectiveness of NIVH in patients with COPD-CRF in the US Medicare population. Analyzing 2012 to 2017 data, we found that NIVH use following a COPD-CRF diagnosis was associated with significant reductions in hospitalizations and ED visits. Further analysis is needed to explore the association between NIVH and all-cause mortality.
Our main analysis results suggest that patients with COPD-CRF receiving NIVH treatment have a 38.3% reduction in risk of death, 21.0% reduction in risk of hospitalizations, and 42.9% reduction in risk of ED visits compared with untreated controls. The reduction in risk of hospitalizations is constant over the study period, whereas the reductions in risk of all-cause mortality and ED visits diminish over time. After 69 weeks, NIVH no longer showed a survival benefit, and after 81 weeks, it no longer showed a reduced risk of ED visits.
The sensitivity analyses included patients receiving NIVH 2 months or more after CRF diagnosis, and no survival benefit was observed. This observation combined with the diminishing survival benefit of NIVH observed in the main analysis suggests that the earlier individuals receive NIVH treatment following CRF diagnosis, the greater the impact on reducing risk of death. Further support of this hypothesis is the observation that patients live longer if NIVH is started earlier following CRF diagnosis. For those receiving NIVH within the first 60 days following diagnosis, median survival is 184 days and mean survival is 268 days. For those starting NIVH more than 60 days following diagnosis, median survival is 132 days and mean survival is 192 days. Further research is needed to explore the association between timing of NIVH treatment and all-cause mortality.
One year after index date, results show an RD for mortality of 13.0% and an RRR for mortality of 30.7% in patients with COPD-CRF receiving NIVH compared with those not receiving NIVH. These findings suggest that NIVH provides a mortality reduction larger than any COPD-CRF treatment outside of smoking cessation.34 The NNT to prevent a death for 1 year was 7.7. This compares favorably with findings from another recently reported COPD treatment. The IMPACT trial reported that treatment with a long-acting muscarinic antagonist/long-acting β antagonist/inhaled corticosteroid inhaler (closed triple therapy) provided a mortality benefit compared with dual-therapy inhalers in a COPD population. In that study, the NNT to prevent a death with triple therapy was greater than 100.35
The 1-year RD and RRR for first hospitalization and first ED visit were clinically meaningful and statistically significant and resulted in an NNT to prevent a hospitalization of 12.0 and an NNT to prevent an ED visit of 5.7. Extrapolation of these results to the full Medicare fee-for-service population suggests that providing NIVH to all patients with COPD-CRF could save as many as 96,000 lives annually while eliminating 62,000 hospitalizations and 130,000 ED visits.
Because 60% of the patients in our study initially received a diagnosis of COPD-CRF while hospitalized, the high mortality rates observed in our sample (7% at 1 week post diagnosis and 47% at 1 year post diagnosis) were expected. These findings are similar to previously reported results in patients receiving NIV while hospitalized for a COPD exacerbation.36 Our findings of a rapid, robust, and persistent reduction in mortality rates following the institution of NIVH in patients with COPD-CRF are also observed in a randomized controlled trial by Köhnlein et al.13
Although our results show significant results in reduced mortality risk, risk of hospitalizations, and risk of ED events, they do not speak to the health-related QOL (HRQOL) of patients receiving NIVH treatment. The literature shows mixed results regarding the effect of NIVH on QOL. Duiverman et al reported that NIVH and NIV improved HRQOL among patients with COPD.37 However, Tissot et al found that HRQOL did not improve among older patients receiving NIVH.38
Limitations
This study has important limitations. Foremost is the concern about making causal inferences from retrospective data. Because randomization was impossible with our design, we employed statistical techniques to control for confounders. Additionally, immortal-time bias is an important concern,33 which we addressed by choosing the shortest possible window of receiving treatment after the index date that provided a treatment group large enough for our analysis to have sufficient power and by performing sensitivity analyses that incorporated time-varying exposure to NIVH.
We used ICD-9-CM and ICD-10-CM codes to identify CRF diagnosis in the Medicare LDS. We were unable to determine whether patients were hypercapnic, hypoxic, or had another form of CRF prior to October 2015 because ICD-9-CM codes do not provide that level of detail. Other studies of this topic have limited NIVH use to hypercapnic patients based on the theory that this group is most likely to benefit. Although this seems rational, no study of NIVH has compared outcomes among different forms of CRF. Our data suggest that clinical benefits are associated with NIVH use in patients with COPD-CRF, but we cannot infer whether one clinical phenotype benefits more than another.
In addition, the Medicare LDS does not include Medicaid dual eligibility status prior to 2017. Medicaid dual eligibility is used as a proxy to adjust for low socioeconomic status in prior studies. As such, this study was unable to control for differences in socioeconomic status between the treatment and control groups.
Furthermore, the findings of this study cannot be extrapolated to the Medicare non–fee-for-service population, such as those in managed care programs or health maintenance organizations, because claims data for these patients are not reported in the Medicare LDS.39
Finally, our study cannot speculate about the level of compliance with NIVH or the optimal settings necessary to achieve improved clinical outcomes. All treated patients in our analysis had NIVH available within 2 months of index diagnosis, but neither the ventilator settings nor the frequency and duration of NIVH use are reported in the Medicare LDS.
CONCLUSIONS
NIVH was associated with lower risks of hospitalizations and ED visits in patients with COPD-CRF. Further research is needed to examine the effect of NIVH on all-cause mortality.
Author Affiliations: VieMed Inc (WF), Lafayette, LA; PRECISIONheor (EvE, RM), Los Angeles, CA; Harvard Medical School (ABJ), Boston, MA.
Source of Funding: Financial support for this research was provided by VieMed Healthcare Inc.
Author Disclosures: Dr Frazier is employed by VieMed Inc as chief medical officer and is also a board member of and stock owner in ViewMed, which is a national supplier of noninvasive ventilation at home; he also attended the American College of Clinical Pharmacy meeting in October 2019. Ms van Eijndhoven is an employee of Precision Health Economics, a consultancy for the health and life sciences industries. Mr Murphy is an employee of Precision Xtract, a health care consultancy firm that performed this research. Dr Jena has received consulting fees unrelated to this work from Pfizer, Bristol Myers Squibb, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals, AstraZeneca, Celgene, Tesaro, Sanofi Aventis, Biogen, Precision Health Economics, and Analysis Group; he has also provided expert testimony through Analysis Group that is unrelated to this study.
Authorship Information: Concept and design (WF, EvE, RM, ABJ); acquisition of data (EvE); analysis and interpretation of data (WF, EvE, ABJ); drafting of the manuscript (WF, EvE, RM); critical revision of the manuscript for important intellectual content (WF, EvE, RM, ABJ); statistical analysis (EvE, ABJ); obtaining funding (WF); administrative, technical, or logistic support (RM); and supervision (WF, EvE, ABJ).
Address Correspondence to: Richard Murphy, BA, PRECISIONheor, 11100 Santa Monica Blvd, Ste 500, Los Angeles, CA 90034. Email: richard.murphy@precisionvh.com.
REFERENCES
1. Basics about COPD. CDC. Updated June 9, 2021. Accessed August 16, 2021. https://www.cdc.gov/copd/basics-about.html
2. Punturieri A, Croxton TL, Weinmann GG, Kiley JP. Chronic obstructive pulmonary disease: a view from the NHLBI. Am J Respir Crit Care Med. 2008;178(5):441-443. doi:10.1164/rccm.200807-1128OE
3. Chronic obstructive pulmonary disease (COPD) includes: chronic bronchitis and emphysema. CDC. Accessed July 20, 2018. https://www.cdc.gov/nchs/fastats/copd.htm
4. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. American Lung Association. March 2013. Accessed July 20, 2018. https://www.lung.org/assets/documents/research/copd-trend-report.pdf
5. Patel JG, Coutinho AD, Lunacsek OE, Dalal AA. COPD affects worker productivity and health care costs. Int J Chron Obstruct Pulmon Dis. 2018;13:2301-2311. doi:10.2147/COPD.S163795
6. Carone M, Antoniu S, Baiardi P, Digilio VS, Jones PW, Bertolotti G; QuESS Group. Predictors of mortality in patients with COPD and chronic respiratory failure: the Quality-of-Life Evaluation and Survival Study (QuESS): a three-year study. COPD. 2016;13(2):130-138. doi:10.3109/15412555.2015.1067294
7. Oga T, Windisch W, Handa T, Hirai T, Chin K. Health-related quality of life measurement in patients with chronic respiratory failure. Respir Investig. 2018;56(3):214-221. doi:10.1016/j.resinv.2018.01.006
8. Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019;54(3):1901003. doi:10.1183/13993003.01003-2019
9. Escalating Medicare billing for ventilators raises concerns. HHS Office of Inspector General. September 22, 2016. Accessed July 20, 2018. https://oig.hhs.gov/oei/reports/oei-12-15-00370.asp
10. Struik FM, Sprooten RTM, Kerstjens HAM, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826-834. doi:10.1136/thoraxjnl-2014-205126
11. Coughlin S, Liang WE, Parthasarathy S. Retrospective assessment of home ventilation to reduce rehospitalization in chronic obstructive pulmonary disease. J Clin Sleep Med. 2015;11(6):663-670. doi:10.5664/jcsm.4780
12. Galli JA, Krahnke JS, Mamary AJ, Shenoy K, Zhao H, Criner GJ. Home non-invasive ventilation use following acute hypercapnic respiratory failure in COPD. Respir Med. 2014;108(5):722-728. doi:10.1016/j.rmed.2014.03.006
13. Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698-705. doi:10.1016/S2213-2600(14)70153-5
14. Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177-2186. doi:10.1001/jama.2017.4451
15. Weir M, Marchetti N, Czysz A, et al. High intensity non-invasive positive pressure ventilation (HINPPV) for stable hypercapnic chronic obstructive pulmonary disease (COPD) patients. Chronic Obstr Pulm Dis. 2015;2(4):313-320. doi:10.15326/jcopdf.2.4.2015.0145
16. Zhou L, Li X, Guan L, et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trial. Int J Chron Obstruct Pulmon Dis. 2017;12:1279-1286. doi:10.2147/COPD.S127540
17. Gantzhorn EK, Prior TS, Hilberg O. Long-term non-invasive ventilation for stable chronic hypercapnic COPD. Eur Clin Respir J.2019;6(1):1644893. doi:10.1080/20018525.2019.1644893
18. Wilson ME, Dobler CC, Morrow AS, et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2020;323(5):455-465. doi:10.1001/jama.2019.22343
19. Medicare Limited Data Set (LDS) quarterly claims and enrollment data. Research Data Assistance Center. September 1, 2016. Accessed July 20, 2018. https://www.resdac.org/articles/medicare-limited-data-set-lds-quarterly-claims-and-enrollment-data
20. Janssens JP, Cicotti E, Fitting JW, Rochat T. Non-invasive home ventilation in patients over 75 years of age: tolerance, compliance, and impact on quality of life. Respir Med. 1998;92(12):1311-1320. doi:10.1016/s0954-6111(98)90135-4
21. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331. doi:10.1164/rccm.200912-1869OC
22. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14(15):1707-1723. doi:10.1002/sim.4780141510
23. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239-241. doi:10.1093/biomet/69.1.239
24. Box-Steffensmeier JM, Jones BS. Event History Modeling: A Guide for Social Scientists. Cambridge University Press; 2004.
25. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761-767. doi:10.1093/aje/kwq439
26. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642-5655. doi:10.1002/sim.7084
27. Deb S, Austin PC, Tu JV, et al. A review of propensity-score methods and their use in cardiovascular research. Can J Cardiol. 2016;32(2):259-265. doi:10.1016/j.cjca.2015.05.015
28. Moretz C, Sharpsten L, Bengtson LG, et al. Real-world effectiveness of umeclidinium/vilanterol versus fluticasone propionate/salmeterol as initial maintenance therapy for chronic obstructive pulmonary disease (COPD): a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:1721-1737. doi:10.2147/COPD.S204649
29. Roberts MH, Mapel DW, Petersen H. Comparative causal analysis of the effects of long-acting muscarinic antagonist versus no long-acting bronchodilator use on readmission or mortality after hospitalization for chronic obstructive pulmonary disease. Drugs Real World Outcomes. 2020;7(1):1-17. doi:10.1007/s40801-019-00171-w
30. Austin PC, Small DS. The use of bootstrapping when using propensity-score matching without replacement: a simulation study. Stat Med. 2014;33(24):4306-4319. doi:10.1002/sim.6276
31. Efron B, Tibshirani RJ. An Introduction to the Bootstrap. CRC Press; 1994.
32. Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63(1):46-55. doi:10.1016/j.jclinepi.2009.03.012
33. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016-1023. doi:10.1093/aje/kwi307
34. Strassmann R, Bausch B, Spaar A, Kleijnen J, Braendli O, Puhan MA. Smoking cessation interventions in COPD: a network meta-analysis of randomised trials. Eur Respir J. 2009;34(3):634-640. doi:10.1183/09031936.00167708
35. Lipson DA, Barnhart F, Brealey N, et al; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi:10.1056/NEJMoa1713901
36. Lindenauer PK, Dharmarajan K, Qin L, Lin Z, Gershon AS, Krumholz HM. Risk trajectories of readmission and death in the first year after hospitalization for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(8):1009-1017. doi:10.1164/rccm.201709-1852OC
37. Duiverman ML, Vonk JM, Bladder G, et al. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial. Thorax. 2020;75(3):244-252. doi:10.1136/thoraxjnl-2019-213303
38. Tissot A, Jaffre S, Gagnadoux F, et al; IRSR NIV Cohort Group. Home non-invasive ventilation fails to improve quality of life in the elderly: results from a multicenter cohort study. PLoS One. 2015;10(10):e0141156. doi:10.1371/journal.pone.0141156
39. Brennan N, Ornstein C, Frakt AB. Time to release Medicare Advantage claims data. JAMA. 2018;319(10):975-976. doi:10.1001/jama.2017.21519

Quality of Life: The Pending Outcome in Idiopathic Pulmonary Fibrosis
February 6th 2026Because evidence gaps in idiopathic pulmonary fibrosis research hinder demonstration of antifibrotic therapies’ impact on patient quality of life (QOL), integrating validated health-related QOL measures into trials is urgently needed.
Read More
A Pulmonologist on Why You Should Think About Respiratory Health and the Lungs
November 16th 2021On this episode of Managed Care Cast, we speak with MeiLan K. Han, MD, MS, the author of a book released this month called Breathing Lessons: A Doctor’s Guide to Lung Health. Han, a pulmonologist, gives an inside tour of the lungs and how they work, zooms out to examine the drivers of poor respiratory health, and addresses policy changes that are needed to improve lung health.
Listen
Ambient AI Tool Adoption in US Hospitals and Associated Factors
January 27th 2026Nearly two-thirds of hospitals using Epic have adopted ambient artificial intelligence (AI), with higher uptake among larger, not-for-profit hospitals and those with higher workload and stronger financial performance.
Read More
Motivating and Enabling Factors Supporting Targeted Improvements to Hospital-SNF Transitions
January 26th 2026Skilled nursing facilities (SNFs) with a high volume of referred patients with Alzheimer disease and related dementias may work harder to manage care transitions with less availability of resources that enable high-quality handoffs.
Read More

