- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
No Significant Differences in Resistance Observed Against 7 Antibiotics in Those With AV
No significant differences in resistance were observed against participants with acne vulgaris (AV) against 7 antibiotics.
Patients with acne vulgaris (AV) with the biofilm-forming (BF) and non–biofilm-forming (NBF) bacteria Cutibacterium acnes (C acnes) and Staphylococcus epidermidis (S epidermidis) had no significant differences in resistance against 7 antibiotics, according to Clinical, Cosmetic, and Investigational Dermatology.
Moreover, some resistances were seen against clindamycin, erythromycin, and azithromycin, but they were not statistically significant.
"The rise of antibiotic resistance in acne has become a major concern in dermatology practice, and the ability of C. acnes and S. epidermidis to form biofilms is believed to contribute to increased antibiotic resistance in acne," the authors explained.
First, the researchers aimed to evaluate the comparison of antibiotic resistance between BF and NBF strains of C. acnes and S. epidermidis towards 7 commonly used antibiotics for acne.
A total of 60 patients with AV were included in this cross-sectional analytical study. Samples were acquired from closed forehead comedones using the standardized skin surface biopsy (SSSB) method at the Cosmetic Dermatology Clinic in Indonesia. Isolates were cultured and identified before the biofilm-forming test was conducted. Each antibiotic underwent susceptibility testing using the disc diffusion method.
Kirby-Bauer Disk Diffusion Antimicrobial Susceptibility Test black background | © Nicolae Adobe Stock Image
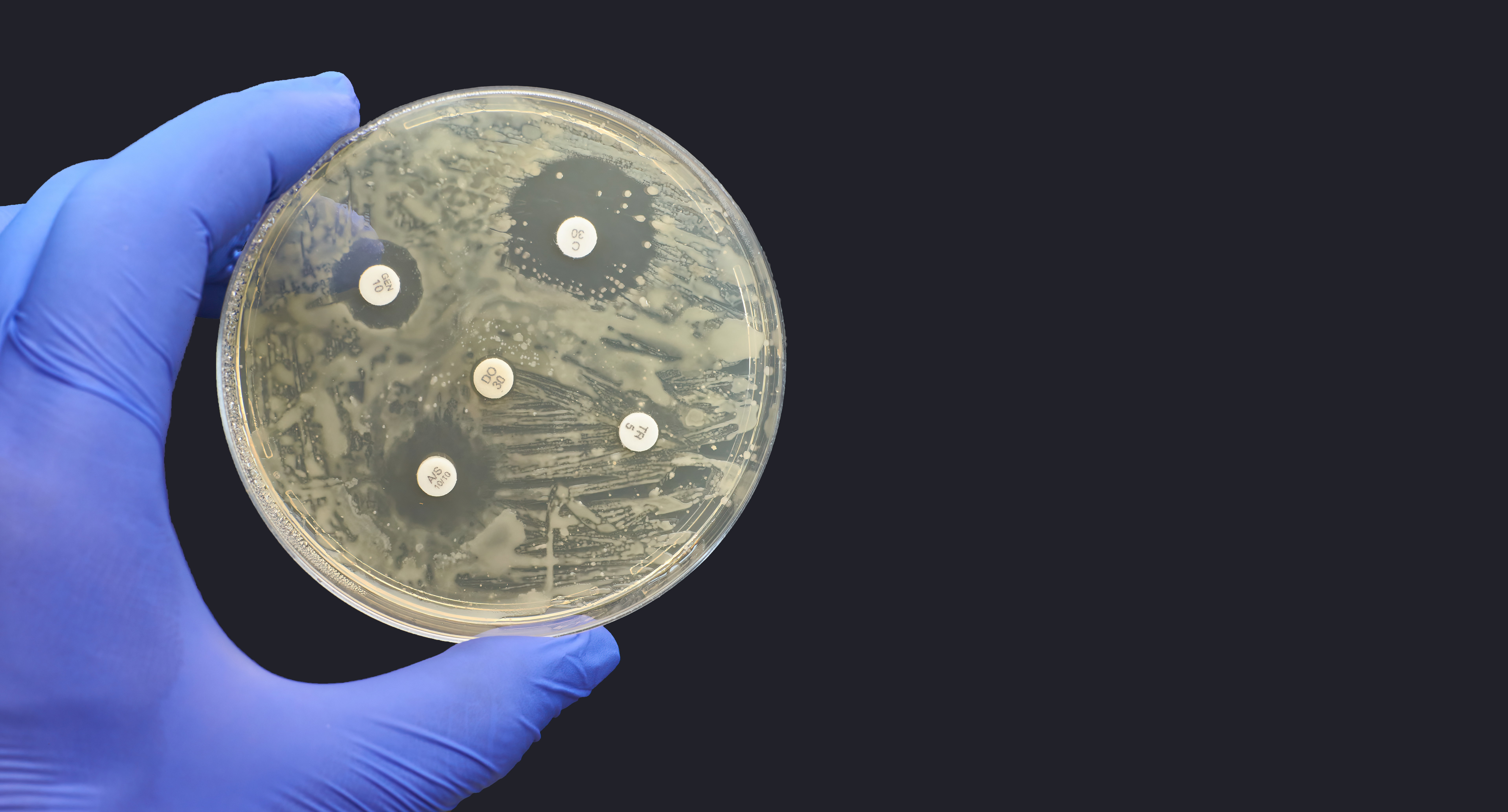
Antibiotic resistance incidence to clindamycin in BF and NBF C acnes isolates was 54.5% (P = 1.00), while in BF S epidermidis isolates it was 54.5% and NBF S epidermidis isolates was 45.5% (both P = .67). Antibiotic incidence resistance to erythromycinin BF and NBF C acnes isolates was 54.5% and azithromycin BF and NBF C acnes isolates was 63.6% (both P = 1.00), whereas for S epidermidis BF and NBF isolates, it was 54.5% (P = 1.00). No resistance was seen to tetracycline, doxycycline, levoflaxcin, and cotrimoxazole across all groups.
This study showed that the BF group of S epidermidis resisted antibiotics more frequently compared with the NBF group.
“On the other hand, in C. acnes strains, the NBF group showed a tendency to be more resistant to antibiotics, although this difference was not statistically significant,” continued the researchers.
The topic is controversial because other authors have reached drastically different conclusions using different approaches and different species of therapeutically relevant bacteria. C acnes and S epidermidis are known biofilm producers. Biofilms offer protection against shear forces, preserve an inflammatory environment in vivo, and support the transformation of C acnes and S epidermidis into metabolically dormant persister cells. The biofilms create an ideal anaerobic environment needed for the growth of C acnes.
“In chronic infections, where C. acnes and S. epidermidis establish long-term persistence within biofilms, the expression of virulence factors is downregulated to accommodate the lower metabolic activity within the [exopolysaccharides]. This can result in therapeutic failure and a decreased quality of life for affected patients,” said the researchers.
Additionally, the chemical composition of biofilms prevents the diffusion of antimicrobials, acting as a pharmokinetic barrier to these treatments. Therefore, the minimum inhibitory concentrations needed to target biofilm-embedded bacteria might be 101 to 104 times higher than those required for planktonic bacteria. The environment has a significant influence on biofilm formation, and the structure of biofilms is extremely dependent on the surrounding environment, suggesting that biofilms adapt to local conditions.
“Based on our experimental data, we did not find any significant differences or notable relationship between biofilm formation and antibiotic resistance,” said the researchers.
Reference
Ruchiatan K, Rizqandaru T, Satjamanggala PR, et al. Characteristics of biofilm-forming ability and antibiotic resistance of cutibacterium acnes and staphylococcus epidermidis from acne vulgaris patients. Clin Cosmet Investig Dermatol. 2023;16:2457-2465. doi:10.2147/CCID.S422486
