- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Impact of Including Drug Spending in Oncology Alternative Payment Models
This study demonstrates a method for understanding the effects of drug spending in the design of alternative payment models.
ABSTRACT
Objectives: To develop a method for determining the effect of including drug costs in alternative payment models (APMs).
Study Design: Retrospective claims analysis.
Methods: Using the Oncology Care Model as an example, we developed an oncology episode payment model for a commercial payer using historical claims data. We defined 6-month episodes of chemotherapy. Using claims data, we characterized episodes and developed a risk adjustment model. We used bootstrapping to estimate the variation in episode cost with drugs included and without.
Results: Episode costs were approximately $100,000. Although absolute cost variation was higher when we included drugs, the percent of total cost represented by variation was lower. Under reasonable assumptions about potential savings from drug and nondrug spending, our results suggest that including drugs in APMs can improve the risk-benefit trade-off faced by provider groups. We introduce a risk-mitigated sharing rate that may enable inclusion of drugs in APMs without substantially increasing downside risk.
Conclusions: We have developed a method to assess whether the inclusion of drug spending in APMs is a good decision for provider groups. Including drug costs in episode payments for oncology patients may be preferable for many provider groups.
Am J Manag Care. 2023;29(11):579-584. https://doi.org/10.37765/ajmc.2023.89454
Takeaway Points
Many providers prefer to exclude drug costs from alternative payment models, particularly when expensive evidence-based drugs exist for the patient population in question. Providers generally do not have control over the cost of drugs. In this article, we develop and demonstrate a method for determining whether inclusion of drug costs is the right decision for providers who are considering alternative payment models.
- Using claims analysis and understanding of care delivery, providers can assess the pros and cons of including drug costs.
- We demonstrate that including drug spending in a cancer chemotherapy bundled payment model may be the best choice for a cancer care provider.
- The method demonstrated in this article can be applied to any type of risk-based payment model and can substitute other specific aspects of the payment model beyond drugs.
Alternative payment models (APMs) for cancer care (eg, Medicare’s Oncology Care Model [OCM] and Enhancing Oncology Model) usually are designed around the total cost of care (TCOC) for a given episode of care. TCOC includes the cost of expensive—but often difficult to substitute—chemotherapy and immunotherapy drugs. These drugs can constitute up to half of the overall spending of many patients with cancer.1,2 Hence, including them in APMs is economically relevant and necessary to align financial incentives with key clinical drivers of cost. Providers, however, are hesitant to take financial accountability for drugs in APMs for several reasons. They believe physicians cannot influence drug prices and utilization,3 and they believe drug spending is volatile and difficult to predict through risk adjustment.4 Providers also invoke the ethical dilemma of profiting from giving patients a less expensive drug. As a result, risk-bearing entities (eg, provider groups and health systems) considering participation in APMs that include drug spending often feel overly exposed to the actuarial risk of treating patients who need high-cost drugs. These concerns have led some to advocate for carving out drug costs from APMs,5 despite limited evidence on the impact of including drug spending in oncology APMs.4
This article provides a methodology to evaluate how including drug spending affects the financial risk-benefit trade-off of a provider group participating in an oncology APM. We focus on 2 key outcomes: (1) the magnitude of expected shared savings from the APM, which a provider group generates by delivering higher-value care (better-quality care at lower cost), and (2) the variation in average payment arising from the patient case mix. Variation in patient cost that cannot be accounted for by risk adjustment exposes provider groups to significant uncontrollable financial risk in an APM. It is unclear to what degree the inclusion of drug spending in an APM affects the balance between these 2 outcomes of interest. On the one hand, including drugs generates a greater savings opportunity by adhering to high-value clinical pathways or utilizing cheaper but equally effective biosimilar or generic drugs. On the other hand, expensive drugs may exacerbate the uncontrollable variation from patient case mix.
Our key contribution is showing how to estimate variation from patient case mix before designing the APM, using historical claims data. Quantifying this variation for a TCOC model with and without drug spending allows stakeholders to understand the impact of including drugs on actuarial risk. This article uses an oncology-based episode payment model as a case study.
METHODS
Conceptual Framework
We contemplate a hypothetical APM with upside shared savings and downside financial risk for systemic cancer therapy episodes. Each episode is assigned a risk-adjusted target payment and attributed to a provider group using preestablished rules. Provider groups earn (or must pay back) the difference between the target payment and the actual episode cost. This reconciliation happens on the aggregate level for all episodes attributed to a participating provider group throughout a set period. Medicare’s OCM is an excellent example, although our framework applies to broader settings.6
The 2 main characteristics of APMs that inform our discussion are the mean shared savings payment, which we denote as B, and the variation in this payment that arises from patient case mix, which we denote as σ. The shared savings payment is the mean amount of shared savings (or losses) a provider group can expect to receive per episode from participating in the APM. Savings can arise by achieving better clinical outcomes (eg, reducing hospital admissions), increasing the efficiency of care delivery, implementing pathways, etc.7 Generally, provider groups that participate in APMs expect to receive a positive shared savings payment on average (if that were not the case, the provider group should not participate in the APM). However, the presence of case-mix variation means that a financial loss is still possible.
Provider groups will prefer APMs with a higher mean shared savings payment B (holding σ fixed), indicating a larger revenue opportunity, or a lower σ (holding B fixed), indicating less uncertainty. We can express the shared savings payment as a function of total spending S and a savings rate r so that B = S × r. The savings rate r can be interpreted as the percentage difference between the target spending total and the realized spending total. We can also break down the total payment across different spending categories. Because we care about drug and nondrug (or medical) spending, we can write
B = rmSm + rdSd,
where the subscripts m and d indicate medical and drug spending, respectively.
This setup allows us to isolate the additional benefit of including drugs in the APM as rdSd. If rd is greater than or equal to 0, an APM based on TCOC will yield a higher mean shared savings payment than an APM that excludes drug spending. However, this APM may also have higher variation, which is a trade-off of uncertain magnitude for a provider group.
Estimation of Episode Case Mix and Variation in Financial Outcome
The primary source of payment variation is patient case mix, which arises from imperfect patient-level risk adjustment. We measure this variation by comparing actual spending with risk-adjusted spending in a data set covering 2 years of commercial claims. We use the first year of data to train a risk-adjustment model that predicts episode spending as a function of several control variables, including the Elixhauser Comorbidity Index score, cancer type, cancer site interacted with surgery status, episode start date, and geographical region of the provider. We use a generalized linear model with a γ distribution and log link to predict spending for episodes that start in the second year. The provider group’s performance benchmark is the mean predicted spending across all episodes attributed to that provider group.
The difference between mean actual spending and the performance benchmark represents the provider group’s outcome. Our primary measure of interest is the variation in a system’s possible outcome. This variation represents the component of spending not captured by the risk adjustment. One can think of a provider group’s outcome as the combination of care changes that result in lower spending plus some variation that arises because the provider groups cannot control the combination of episodes they manage in any given year. A provider group will perform poorly if it receives many high-spending episodes relative to the benchmark and vice versa. We cannot observe this variation directly in the data because we observe only 1 outcome for each system. Instead, we estimate it using statistical methods.
We use bootstrapping to estimate the SE (variation) in a provider group’s outcome. Bootstrapping consists of randomly drawing many groups of episodes from those attributed to a given system. Each group represents a hypothetical distribution of episodes that could have been attributed to the system. For example, suppose that a system was attributed 5 high-spending patients and 5 low-spending patients. Under slightly different circumstances, that system may have been attributed 6 high-spending patients and 4 low-spending ones or vice versa. Bootstrapping takes those scenarios into account. We repeat the steps required to estimate this SE using all claims associated with an episode (for TCOC) and then using only nondrug claims (for no drugs).
Using Estimates of Variation to Assess the Viability of a TCOC Model
The additional potential upside of the TCOC model comes from the savings from drug spending: rdSd. If rd is less than or equal to 0—that is, it is impossible to reduce drug spending in cancer care—then the model that excludes drugs is a clear winner: It has the same or higher shared savings payment and lower variation. However, it is reasonable to assume that rd is greater than 0.7,8 Hence, in a symmetrical, 2-sided risk arrangement, the TCOC model has more upside and risk, making the comparison less straightforward.
We propose a simple exercise to obtain a more intuitive comparison. Consider a TCOC contract that limits upside and downside risk by a fraction α. If a system participates in this contract, it will earn a fraction α of the savings but only pay back a fraction α of potential losses. A contract of this type reduces the mean shared savings payment and the risk because the provider group is partially protected against an unlucky draw in episode case mix.
If we set the parameter α as
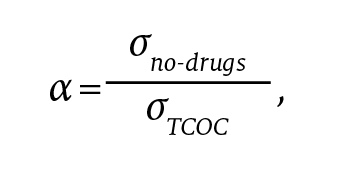
the mitigated-risk contract will earn, on average, α(rmSm + rdSd) and have variation ασTCOC = σno-drugs. Because the variation is the same as the model without drugs, we can simply compare the shared savings payment to determine which model is preferable.
The mitigated-risk TCOC model is preferable as long as
α(rmSm + rdSd) > rmSm.
In this article, we show how one can derive α by estimating σno-drugs and σTCOC even before implementing an APM. To choose an APM design, a provider group can use α along with predictions of rm and rd.
Data Source
We apply our method to a data set of claims between September 2014 and September 2018 for patients aged 18 to 64 years who were covered by a large commercial insurer. We restrict our attention to patients with a chemotherapy or immunotherapy claim (henceforth, qualifying claim) and create 6-month episodes of oncology care that mimic the criteria for inclusion in Medicare’s OCM. Episodes start on the date of the first qualifying claim. We attribute each episode to the provider group that bills the first qualifying claim unless that provider group bills fewer than 25% of all qualifying claims. In that case, we assign the episode to the group billing the plurality of claims. We use the first 6 months of our data to screen for new patients, so our first episodes of care start in March 2015. We cap the spending of episodes with a TCOC over $250,000. We only include spending on chemotherapy and immunotherapy drugs among drug costs. Spending on nononcology drugs represents a small fraction of overall spending, and we include it with medical spending. We use years 2 and 3 of the data (eg, episodes starting between March 2016 and March 2018) in the main results we present here because they are the most recent ones (replicating the analysis using years 1 and 2 does not affect the results).
RESULTS
Mean Spending and Risk Adjustment
Figure 1 plots the mean spending of episodes in our sample, inclusive of the insurer’s cost and out-of-pocket spending. The mean episode cost decreases over time. Oncology-related drug spending remains more or less constant throughout this period, but it increases as a fraction of overall spending. In the last year of our data, spending on chemotherapy and immunotherapy drugs is roughly equivalent to spending on all other categories.
To assess the validity of our risk-adjustment model, we predict spending for all year 3 episodes (n = 3358) and then regress realized spending on predicted spending (Table 1). The results demonstrate that, consistent with providers’ assessment, overall spending is more challenging to predict than nondrug spending. This is reflected in the lower R2 number associated with regressions whose dependent variable is TCOC inclusive of drug spending.
Bootstrap Analysis
We conduct the bootstrap analysis separately by provider group and using only provider groups with at least 15 attributed episodes. For the largest provider group (group A in Table 2) in our data (525 episodes), the analysis shows that the variation of outcomes for this provider group is $1586 when excluding drugs and $2129 when including drugs: a 34% increase (Table 2). The mean spending of an episode also increases. Mean nondrug costs per episode amount to $49,129 but increase to $79,855 when including drugs (a 62% increase). Hence, variation falls as a fraction of overall spending.
We find that this pattern holds regardless of the number of episodes attributed to a group. As groups become smaller in size, variation grows (as seen in the increasing size of σ in the lower rows of Table 2). However, variation grows more or less proportionally in both the TCOC and the no-drug models. We confirm this result by repeating the bootstrapping exercise for group A but reducing the number of draws. This allows us to simulate provider groups with the same patient pool as group A but different sizes. Figure 2 displays the variation in system performance under the TCOC model and the model excluding drugs. As expected, variation increases for simulated groups with smaller sizes. However, the main result holds: Variation in savings when including drug spending is a higher dollar amount (panel A) but represents a lower fraction of total spending included in the model (panel B).
Estimating the Viability of a TCOC Model
After running the bootstrap and understanding the spending and variation left in the sample after risk adjustment, we can assess the conditions under which a provider group will prefer including drugs in the model. Once again, we use group A as an example. For group A, α is approximately equal to 0.75. We can use α to obtain a threshold rule for including drugs in the APM that compares the savings rates on drug and nondrug spending with estimates from our data:
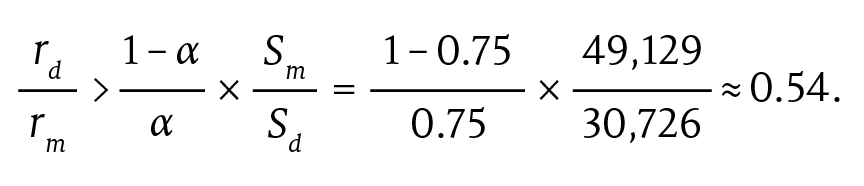
The implication is that if the savings rate from drug spending is a little above half that from nondrug spending, the TCOC mitigated-risk model is preferable to a model that excludes drugs altogether.
We repeat these calculations for each provider group in our data as a robustness check. The last column of Table 2 summarizes the results of this exercise. For most of our provider groups, we find that a ratio of drug to nondrug savings rate between 0.4 and 0.8 would justify including drug spending in an oncology APM. The size-weighted mean ratio across provider groups is approximately 0.61. We find no correlation between the ratio of savings rates and provider group size.
Table 3 explains the implications of different savings rates on the choice of model. We simulate the mean shared savings payment under the TCOC, TCOC without drugs, and mitigated-risk TCOC models for group A under the assumption that the nondrug savings rate rm is 4% and the drug savings rate rd varies between 1% and 4%. For completeness, we also include the range of possible savings defined by adding and subtracting σ from the mean outcome. Under these assumptions, including drugs is advantageous in rows 3 and 4.
DISCUSSION
In this article, we use commercial claims data to quantify the trade-offs between the shared savings opportunity and financial uncertainty of including drugs in an episodic oncology APM. Using bootstrapping methodology, we demonstrate how the balance of these trade-offs hinges on estimates of variation in risk-adjusted spending with and without drugs (σTCOC and σno-drugs) as well as savings expectations on drugs (rd) and medical spending (rm).
Our article contributes to the discussion of the trade-offs between including and excluding drug spending in oncology payment models. Little empirical work exists on the topic. Ward et al simulate providers of different sizes to examine the impact of including drug spending in bundled payment models, but their results rely entirely on simulated data.4
These discussions are becoming more critical in the face of mounting evidence that APMs can significantly affect provider drug prescription behavior.9,10 The stakes are exceptionally high in oncology because of the increasing importance of drug spending, which is driven by novel expensive cancer therapies.11 Our paper provides an objective framework to weigh the increased earnings opportunity against the increased actuarial risk in APMs that include drug spending.
Limitations
We note 3 limitations. First, patient case-mix variation is not constant but likely fluctuates from year to year. Our estimates of σTCOC and σno-drugs are accurate representations of variation in the current year but cannot account for future changes in the market. For example, our method accounts for the impact of new drugs on σTCOC. However, the number of new drugs changes from year to year, which implies that σTCOC would also adjust: upward if more drugs are introduced and downward if fewer drugs are introduced.
Second, our method does not provide a way to predict the savings rate from medical costs or drugs. This would depend on educated guesses from provider experts and/or estimates from the literature. A more accurate but also more expensive solution is to design a 2-phase APM and observe rd and rm in the first phase. The best way to do so would be to implement a TCOC model in the first year but pay provider groups a lump sum sign-up bonus to buffer any potential downside induced by the inclusion of drug spending. This transfer can be benchmarked using the estimates for σno-drugs and σTCOC. The payment protects participating systems from risk while at the same time preserving the incentives of the 2-sided model. Mean savings from the first year can be used to estimate rd and rm.
Finally, although providing a valuable proof of concept, the estimates from our empirical exercise likely have limited external validity. Medicaid and Medicare payment models will follow the same math, but the range of estimates of σ may differ based on different patient populations and the wide variation in commercial reimbursement rates. Moreover, our model used drug costs as reported on claims data. These costs do not reflect rebates and other concessions that manufacturers negotiate with payers.12,13 Although these rebates will not alter the implications or the validity of our approach, they may affect the level of α.
CONCLUSIONS
Variation in the cost of chemotherapy and immunotherapy drugs represents a steep hurdle to the adoption of oncology APMs. This paper introduces a methodology to assess the risk-benefit trade-off of including drugs in oncology APMs. Our main contribution is to provide a concrete and straightforward framework for thinking about the role of drugs and evaluating whether it would benefit providers if drugs were included in the TCOC calculation under APMs. We also conduct a proof-of-concept empirical exercise by applying our methodology to a data set of commercially insured patients.
Finally, although the role of drug spending in oncology APMs has been the focus of much debate recently, we note that our methodology can also be applied more broadly to capitation-based payment models outside oncology and categories of spending beyond drugs. Thus, we provide a general framework to assess the impact of any spending category on the risk-benefit trade-off of any APM centered around bundled payments or capitation.
Author Affiliations: Department of Health Care Policy, Harvard Medical School (LM), Boston, MA; Department of Economics (JPK), Department of Urology (DCJ), Department of Health Policy and Management (GMH), Department of Medicine (DAD), and Cecil G. Sheps Center for Health Services Research (LM, GMH, DAD), University of North Carolina at Chapel Hill, Chapel Hill, NC; Rubicon Founders (DCJ), Nashville, TN.
Source of Funding: Blue Cross and Blue Shield of North Carolina.
Author Disclosures: Dr DeWalt reports funding from Blue Cross and Blue Shield of North Carolina, which designs payment models and contracts with provider systems. The remaining authors report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (LM, DCJ, GMH, DAD); acquisition of data (DCJ, DAD); analysis and interpretation of data (LM, JPK, DCJ, GMH, DAD); drafting of the manuscript (LM, JPK, DCJ, DAD); critical revision of the manuscript for important intellectual content (LM, DCJ, GMH, DAD); statistical analysis (LM, JPK, GMH); obtaining funding (DAD); administrative, technical, or logistic support (DAD); and supervision (LM, DAD).
Address Correspondence to: Darren A. DeWalt, MD, MPH, University of North Carolina at Chapel Hill, 5045 Old Clinic Building, CB 7110, Chapel Hill, NC 27599. Email: dewaltd@med.unc.edu.
REFERENCES
1. Zaorsky NG, Khunsriraksakul C, Acri SL, et al. Medical service use and charges for cancer care in 2018 for privately insured patients younger than 65 years in the US. JAMA Netw Open. 2021;4(10):e2127784. doi:10.1001/jamanetworkopen.2021.27784
2. Keating NL, Jhatakia S, Brooks GA, et al; Oncology Care Model Evaluation Team. Association of participation in the Oncology Care Model with Medicare payments, utilization, care delivery, and quality outcomes. JAMA. 2021;326(18):1829-1839. doi:10.1001/jama.2021.17642
3. Dreyer T, Wilfong LS, Patel K, Gamble B, Polite BN. Oncology Care Model: a Herculean effort with fixable fatal flaws. JCO Oncol Pract. 2021;17(4):173-176. doi:10.1200/OP.20.00852
4. Ward JC, Levit LA, Page RD, et al. Impact on oncology practices of including drug costs in bundled payments. J Oncol Pract. 2018;14(5):e259-e268. doi:10.1200/JOP.17.00036
5. Thomas CA, Ward JC. The Oncology Care Model: a critique. Am Soc Clin Oncol Educ Book. 2016;35:e109-e114. doi:10.1200/EDBK_156883
6. Kline RM, Bazell C, Smith E, Schumacher H, Rajkumar R, Conway PH. Centers for Medicare and Medicaid Services: using an episode-based payment model to improve oncology care. J Oncol Pract. 2015;11(2):114-116. doi:10.1200/JOP.2014.002337
7. Hertler A, Chau S, Khetarpal R, et al. Utilization of clinical pathways can reduce drug spend within the Oncology Care Model. JCO Oncol Pract. 2020;16(5):e456-e463. doi:10.1200/JOP.19.00753
8. Kreys ED, Koeller JM. Documenting the benefits and cost savings of a large multistate cancer pathway program from a payer’s perspective. J Oncol Pract. 2013;9(5):e241-e247. doi:10.1200/JOP.2012.000871
9. Bekelman JE, Gupta A, Fishman E, et al. Association between a national insurer’s pay-for-performance program for oncology and changes in prescribing of evidence-based cancer drugs and spending. J Clin Oncol. 2020;38(34):4055-4063. doi:10.1200/JCO.20.00890
10. Eliason PJ, Heebsh B, League RJ, McDevitt RC, Roberts JW. The effect of bundled payments on provider behavior and patient outcomes: evidence from the dialysis industry. Duke University. February 2022. Accessed October 2, 2023. https://people.duke.edu/~rcm26/DialysisBundle.pdf
11. Martin AB, Hartman M, Washington B, Catlin A. National health care spending in 2017: growth slows to post-Great Recession rates; share of GDP stabilizes. Health Aff (Millwood). 2019;38(1):96-106. doi:10.1377/hlthaff.2018.05085
12. Hernandez I, San-Juan-Rodriguez A, Good CB, Gellad WF. Changes in list prices, net prices, and discounts for branded drugs in the US, 2007-2018. JAMA. 2020;323(9):854-862. doi:10.1001/jama.2020.1012
13. Kakani P, Chernew M, Chandra A. Rebates in the pharmaceutical industry: evidence from medicines sold in retail pharmacies in the U.S. National Bureau of Economic Research working paper No. 26846. March 2020. Accessed October 2, 2023. https://www.nber.org/papers/w26846

Ambient AI Tool Adoption in US Hospitals and Associated Factors
January 27th 2026Nearly two-thirds of hospitals using Epic have adopted ambient artificial intelligence (AI), with higher uptake among larger, not-for-profit hospitals and those with higher workload and stronger financial performance.
Read More

