- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Guselkumab Shows High Efficacy in Patients With Psoriasis, History of Cancer
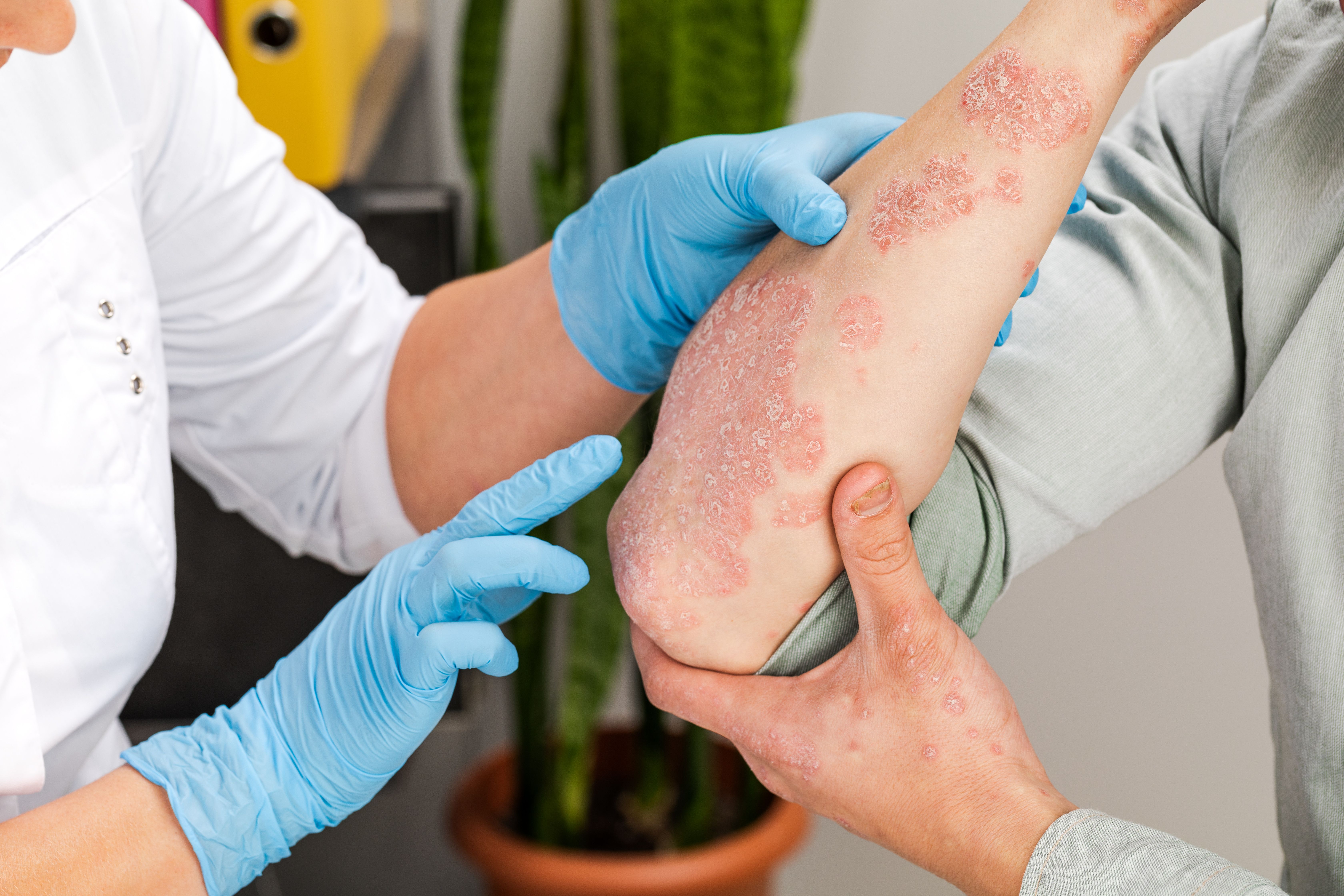
Patients with moderate to severe psoriasis and a history of cancer have improved symptoms and a favorable safety profile with guselkumab treatment, according to a new study.
Guselkumab demonstrated high efficacy and a favorable safety profile in patients with moderate to severe psoriasis and a history of cancer.
This multicenter, retrospective Spanish study is published in Journal of the American Academy of Dermatology International.
“Patients with psoriasis and a personal history of malignancy constitute a unique population, often excluded from clinical trials due to safety concerns and the need for a more cautious approach to their care,” wrote the researchers of the study. “Recognizing the dearth of evidence in this specific context, our study aims to contribute to the body of knowledge by reporting a real-life multicenter experience involving a cohort of patients with moderate to severe psoriasis undergoing treatment with guselkumab and with a history of cancer.”
The study included individuals 19 years and older with moderate to severe psoriasis who had been treated with guselkumab, the first interleukin-23 (IL-23) subunit p19 inhibitor human monoclonal antibody approved in Europe and the US. Additionally, patients included in the study had a diagnosis of neoplasm in the last 5 years or less and had a follow-up period of at least 12 weeks.
Sociodemographic and clinical characteristics included in the analysis were age, sex, years with psoriasis, psoriasis type, psoriasis area and severity index (PASI), body surface area, dermatology life quality index, and visual analog scale pruritis scores. Additionally, the researchers noted any neoplasm variables, including years since diagnosis, type of neoplasm, treatment received, active or in remission, and neoplasm stage.
Twenty patients were included in the study. All patients were treated with guselkumab, with 14 patients having active cancers. Additionally, mean years elapsed since psoriasis diagnosis was 24.3 years, while the mean time from cancer diagnosis was 3.1 years.
The analysis included a 52-week follow-up period to evaluate efficacy and safety, with a mean follow-up of 36 weeks. The percentage of patients who achieved a PASI score of 3 or less was 80% at week 12 and 87.5% at week 52. Additionally, 68.8% of patients achieved a PASI of 1 or less.
The 52-week survival rate was 100%, including the 14 patients with concomitant active cancers. No adverse effects or dropouts related to guselkumab treatment were observed.
However, the researchers acknowledged some limitations to the study, including a relatively small sample size, heterogeneity in cancer types, the inclusion of early-stage tumors, and a short duration of observation. Additionally, only a small number of malignancy cases were observed among patients treated with guselkumab. Furthermore, the researchers noted that the treatment of psoriasis in patients with a history of cancer remains a challenge when it comes to clinical decision-making.
Despite these limitations, the researchers believe the study provides real-world evidence of guselkumab as an effective and safe treatment in patients with moderate to severe psoriasis and a history of cancer.
“It is imperative to foster collaboration between dermatologists, oncologists, and researchers to conduct rigorous studies that address the specific concerns of psoriasis patients with a history of cancer,” wrote the researchers. “As we advance in our understanding of the connections between psoriasis, cancer, and novel therapies, we strive to provide patients with the most effective and evidence-based treatments while ensuring their overall well-being and quality of life.”
Reference
Gracia Cazaña T, Riera Monroig J, Izu R, et al. Real-world outcomes in patients with malignancy and moderate-severe psoriasis treated with guselkumab. JAAD International. Published online April 7, 2024. doi:10.1016/j.jdin.2024.02.019
