- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Excision Bio Seeks to Suppress HIV Replication With CRISPR Gene Therapy
Excision BioTherapeutics has so far released positive safety data from the first 3 participants living with HIV-1, with no evidence of vector shedding in sexual organ tissue.
CGTLive™ first published this article. This version has been lightly edited.
A cure for HIV-1 becomes more feasible as advances continue in gene therapy research and more trials open investigations on potential novel treatments. One such approach among the frontrunners developing a novel therapy for treating HIV-1 is Excision BioTherapeutics’ EBT-101.
EBT-101 uses the CRISPR/Cas9 system, which was recently publicly validated with the FDA approval of Vertex Pharmaceuticals' and CRISPR Therapeutics’ exagamglogene autotemcel (exa-cel [Casgevy]; Vertex Pharmaceuticals), which uses the same gene-editing mechanism.1
Exa-cel is an autologous cell therapy edited with the use of CRISPR/Cas9 before reinfusion. EBT-101 is a multiplexed in vivo CRISPR-based gene-editing therapy that delivers CRISPR/Cas9 and dual-guide RNAs via an adeno-associated virus (AAV), targeting 3 sites in the HIV genome for excision, with the intention of minimizing viral escape.
“Having spoken to some clinicians, they realized that only a handful of people in the world have been cured of HIV ever, so they understand the transformative nature of the therapy. And we believe that the trial will provide important information on the path to a potential functional cure for people living with HIV and meets an area of high unmet medical need. It’s really exciting that we have this groundbreaking therapy for infectious diseases," said TJ Cradick, PhD, chief scientific officer, Excision BioTherapeutics.
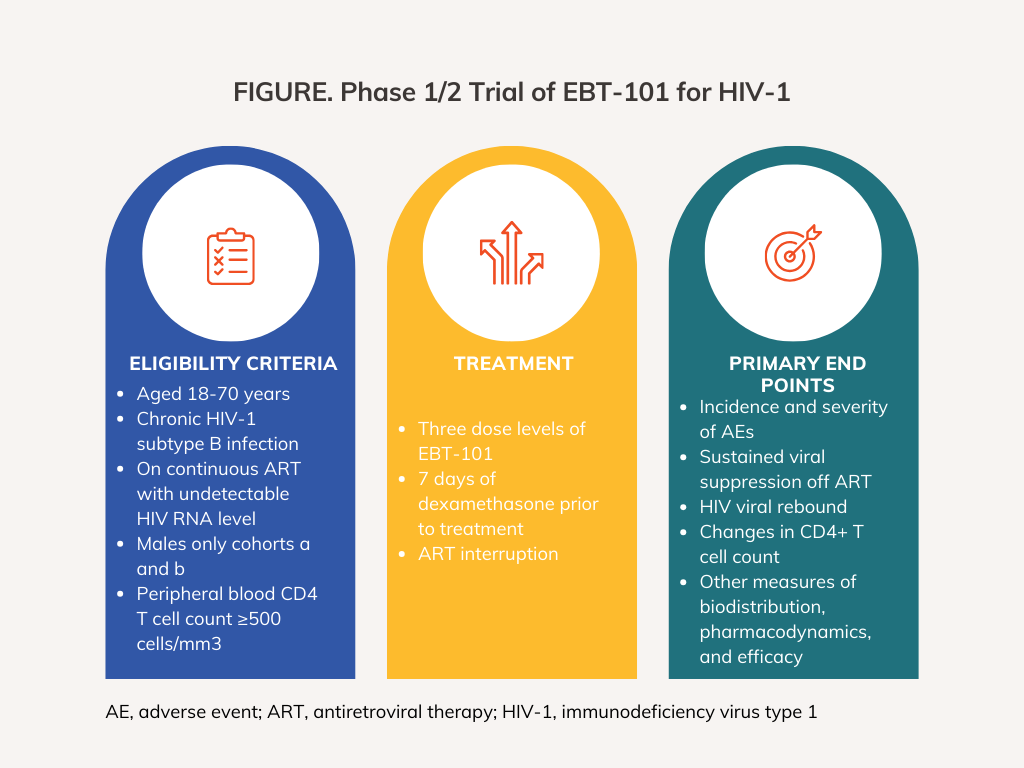
EBT-101 is being evaluated in an open-label, multicenter, phase 1/2 clinical trial (NCT05144386) that has a planned enrollment of 9 male patients aged 18 to 60 years with documented chronic HIV-1 infection. Participants must be on stable HIV suppression via antiretroviral treatment (ART) with a CD4+ T-cell count above 500 cells/mcL for at least 1 year and low anti-AAV9–neutralizing antibody titers. Patients must weight between 45 and 90 kg (inclusive) and be vaccinated against Neisseria meningitidis and SARS-CoV-2.
Patients with drug resistance to 2 or more classes of ART within the past 5 years, with more than 1 change in ART due to virologic failure in the past 2 years, receiving long-acting injectable ART, and who have anti-AAV9–neutralizing antibodies greater than 1:20 titer will be excluded from the study. Patients with a history of HIV dementia or HIV-related cardiac disease, HIV-related kidney disease with abnormal renal function, or HIV-related opportunistic infections within the past 2 years will also be excluded. Additional exclusion criteria relate to past treatments and health status.
Participants receive 7 days of immunosuppression with dexamethasone starting 24 hours before intravenous (IV) EBT-101 administration of 1 of 3 dose-levels, depending on their cohort. They will be followed for up to 48 weeks post treatment and will be assessed for sustained viral suppression off ART in an analytical treatment interruption starting at week 12, with weekly assessments for adverse events, HIV viral rebound, and changes in CD4+ T-cell count. Other measures of biodistribution, pharmacodynamics, and efficacy also will be evaluated.
The study is taking place at multiple locations in the United States and is expected to be completed in March 2025. Participants will also be enrolled in a long-term follow-up study (NCT05143307), which will follow them for up to 15 years.
The trial dosed its first patient in September 2022 and was granted fast track designation by the FDA in July 2023.2,3 The most recent data from the trial, on the first 3 treated patients, were released in October 2023 at the European Society of Gene & Cell Therapy (ESGCT) meeting.4
These participants, treated in cohort A at the first dose level, had no serious adverse effects (AEs) or dose-limiting toxicities. There were 4 mild AEs possibly or definitely related to EBT-101, which resolved without intervention, and 12 mild AEs. There were no infusion-related reactions or withdrawals during IV administration, and no complement-mediated toxicity. Two participants had transient and reversible transaminase elevations. EBT-101 was detectable in blood at 4 weeks in every participant with peripheral exposure. There has been no evidence of horizontal transmission of gene vector shedding in 2 tissue compartments associated with male reproductive function. Excision plans to dose escalate to the 3.0 x 1012 vg/kg dose in the fourth quarter of 2023, with more data to come in 2024.
“A major barrier to curing HIV is viral latency, which is defined as the persistence of integrated proviral DNA that is replication competent. Although ART effectively suppresses viral replication and prevents disease progression to AIDS, long-term treatment with ART does not eliminate latent HIV and people living with HIV on suppressive ART have significant ART-related side effects and non-AIDS–related comorbidities and malignancies,” said Rachel M. Presti, MD, PhD, professor of medicine and medical director, Infectious Disease Clinical Research Unit, Washington University School of Medicine, St. Louis, during her presentation of the data at ESGCT.4
References
1. FDA approves first gene therapies to treat patients with sickle cell disease. News release. FDA. December 7, 2023. Accessed December 18, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
2. Excision Biotherapeutics doses first participant in EBT-101 phase 1/2 trial evaluating EBT-101 as a potential cure for HIV. News release. GlobeNewswire. September 15, 2022. Accessed December 18, 2023. https://www.globenewswire.com/news-release/2022/09/15/2516733/0/en/Excision-BioTherapeutics-Doses-First-Participant-in-EBT-101-Phase-1-2-Trial-Evaluating-EBT-101-as-a-Potential-Cure-for-HIV.html
3. Excision BioTherapeutics receives FDA fast track designation for EBT-101, a first-in-class CRISPR-based gene therapy candidate to functionally cure HIV-1. News release. Yahoo Finance. July 20, 2023. Accessed December 18 , 2023. https://finance.yahoo.com/news/excision-biotherapeutics-receives-fda-fast-110000749.html
4. Presti R, Baxter J, Sivapalasingam S, Gordon J, Gordon TJ, Kennedy WP. First-in-human trial of systemic CRISPR-Cas9 multiplex gene therapy for functional cure of HIV. Presented at: 30th Annual ESGCT Congress; October 24-27, 2023; Brussels, Belgium.
