- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Data Show Trend Toward Improved Persistence After 6 Months With Oral MDS Regimen
The authors concluded that the data support consideration of the oral regimen of decitabine and cedazuridine to reduce the treatment burden associated with intraveous or subcutaneous hypomethylating agents in patients with myelodysplastic syndromes (MDS).
New results show that the oral hypomethylating agent (HMA) decitabine and cedazuridine (DEC-C), approved for treatment of myelodysplastic syndromes (MDS) in July 2020, may improve persistence beyond the 6-month mark compared with HMA agents given intravenously (IV) or subcutaneously (SC).
Real-world data presented in an abstract at the 65th American Society of Hematology Annual Meeting and Exhibition in San Diego show “comparable persistence” between the different formulations in the early stages of therapy, but that by the 6-month mark, a persistence advantage emerged with the oral regimen that increased in later months.
While the trend favored the oral regimen, the authors said it did not reach statistical significance.
ASH 2023 Abstract 548 | Image credit: American Society of Hematology
The authors said theirs was the largest study of real-world data to date involving the oral MDS regimen. The retrospective analysis used data from the US Cerner Enviza claims database, with medical and prescription claims linked to mortality data. The time period covered patients who had an MDS diagnosis and initiated at least 1 claim between August 1, 2020, and August 31, 2022; the first claim served as the index date for the study. Patients were followed until the end of enrollment, death, or the end of the study. Investigators used propensity score matching to account for differences between the groups in age, gender, subsequent diagnosis with acute myeloid leukemia (AML), and red blood cell transfusions.
According to the authors, longitudinal persistence was measured by the number of cycles of therapy received during follow-up, with a cycle defined as either 3-10 days for receipt of IV/SC treatment or 1 claim for DEC-C, within each 28-day cycle.
Results. The analysis began with 1569 patients, including 160 (10.2%) who received DEC-C and 1409 (89.8%) who received IV/SC HMAs. Patients in the DEC-C group had a median age of 72.5 years and 66.9% were male. In the IV/SC group, the median age was 69 years and 58.2% were male. More than two-third of patients in both groups had switched from a prior IV/SC HMA treatment, and the groups were well-balanced for levels of comorbidity; in the 6 months prior to their index date, 55.3% of patients in both groups received transfusions.
After matching, there were 158 patients in each treatment group. Median age was 72 years in the DEC-C group and 74 years in the IV/SC group. The groups were 66.5% and 69.6% male, respectively. Rates for comorbidities and AML diagnoses were comparable.
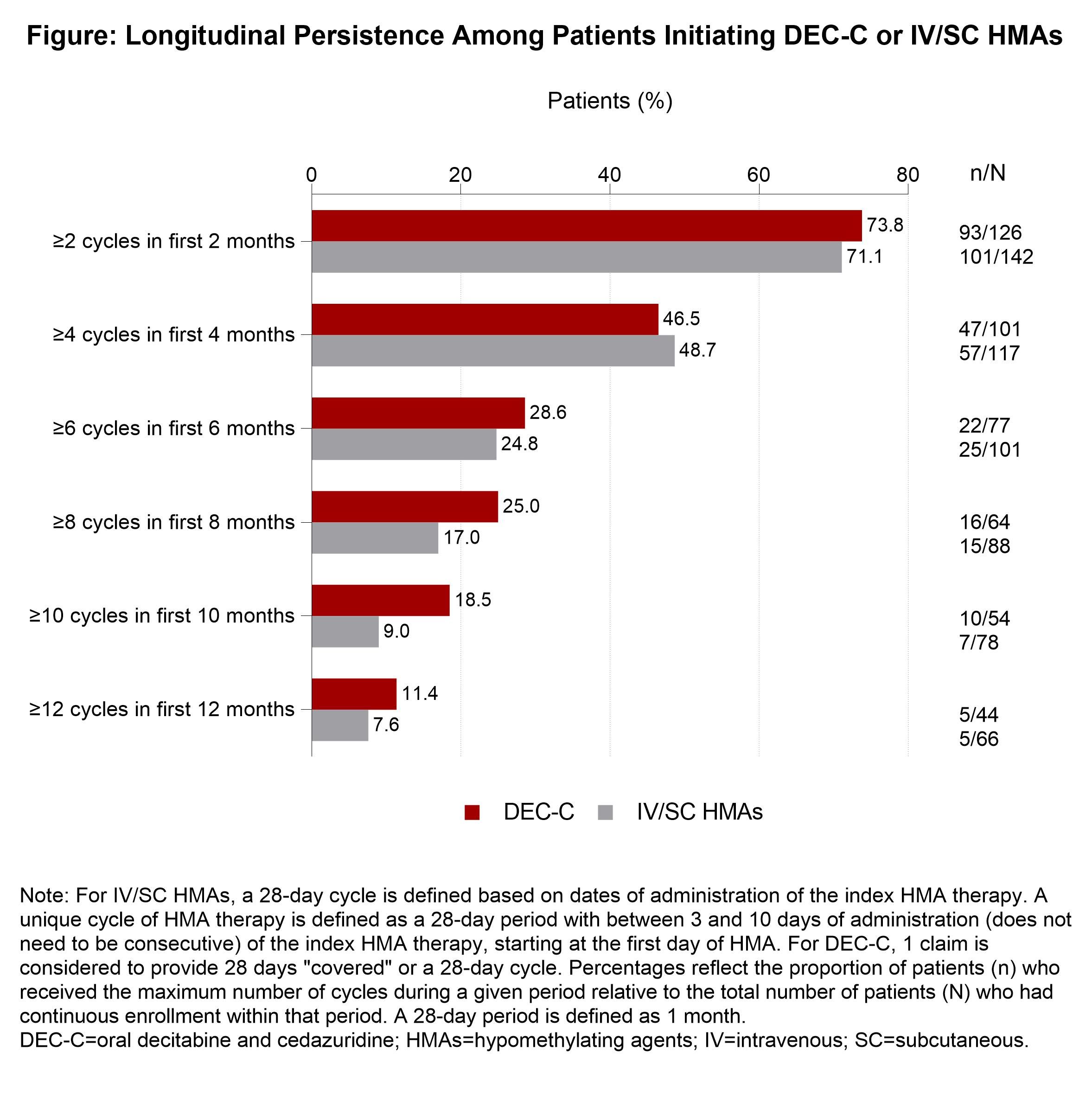
During the first 6 months after the index date, persistence was comparable between the 2 groups, with similar shares of each group receiving the maximum number of treatment cycles: at 2 months, it was 73.8% for DEC-C vs 71.1% for IV/SC; at 4 months, 46.5% vs 48.7%; and at 6 months, 28.6% vs 24.8%.
After that, the authors wrote, “a trend towards improved persistence with DEC-C versus IV/SC HMAs was observed,” with the oral regimen pulling away: at 8 months, 25.0% vs 17.0%; at 10 months, 18.5% vs 9.0%; at 12 months, 11.4% vs 7.6%. The number of days to discontinuation of treatment was higher for the oral regimen group compared with the IV/SC treatment group, at 87.7 days vs 82.0 days. Neither of these differences reached statistical significance.
Nonetheless, the authors concluded: “These data support the consideration of oral DEC-C as an alternative to chronic parenteral HMA therapy, with the potential for oral DEC-C to reduce the treatment burden associated with the administration of IV/SC HMAs.”
Oral decitabine and cedazuridine is marketed as Inqovi by Taiho Oncology, which provided funding for the study.
Reference
Zeidan AM, Costantina H, Modi K, Salimi T, Washington T, Epstein RS. Real-world treatment patterns among patients with myelodysplastic syndromes initiating oral decitabine and cedazuridine or intravenous/subcutaneous hypomethylating agents. Presented at: 65th American Society of Hematology Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. Abstract 548. https://ash.confex.com/ash/2023/webprogram/Paper188638.html
The Importance of Examining and Preventing Atrial Fibrillation
August 29th 2023At this year’s American Society for Preventive Cardiology Congress on CVD Prevention, Emelia J. Benjamin, MD, ScM, delivered the Honorary Fellow Award Lecture, “The Imperative to Focus on the Prevention of Atrial Fibrillation,” as the recipient of this year’s Honorary Fellow of the American Society for Preventive Cardiology award.
Listen
