- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Deep Learning Biomarkers Help Uncover ENV-101 Treatment Effects in IPF
At 12 weeks, patients treated with ENV-101 showed statistically significant increases in lung volume compared with those receiving placebo.
Novel CT-based tools detected improvements in lung volume and fibrosis in patients with idiopathic pulmonary fibrosis (IPF) treated with Hedgehog pathway inhibitor ENV-101, according to research presented at the American Thoracic Society (ATS) 2025 International Conference.1
IPF is a progressive, fatal lung disease characterized by scarring and declining lung function, with a median survival of just 3 years.2 Standard imaging lacks sensitivity to subtle disease changes, making it difficult to monitor treatment response. In this study, investigators used artificial intelligence (AI) models to extract detailed quantitative measures from high-resolution CT (HRCT) scans, offering a potentially more responsive way to evaluate treatment effects.1
AI-Based Imaging of Disease Progression
The analysis used data from ENV-IPF-101 (NCT04968574), a double-blind, placebo-controlled phase 2a trial evaluating ENV-101 in adults with IPF.3 ENV-101, developed by Endeavor BioMedicines, inhibits aberrant Hedgehog pathway signaling, a key driver of myofibroblast activation and lung fibrosis.1 Participants were randomized 1:1 to receive either ENV-101 or placebo once daily for 12 weeks.
For this post hoc analysis, researchers from Qureight and Endeavor BioMedicines analyzed paired HRCT scans from 34 patients—16 on treatment and 18 on placebo—using 4 validated deep learning models:
ENV-101 demonstrated a significant reduction in pulmonary vascular volume compared with placebo. | Image credit: shidlovski – stock.adobe.com
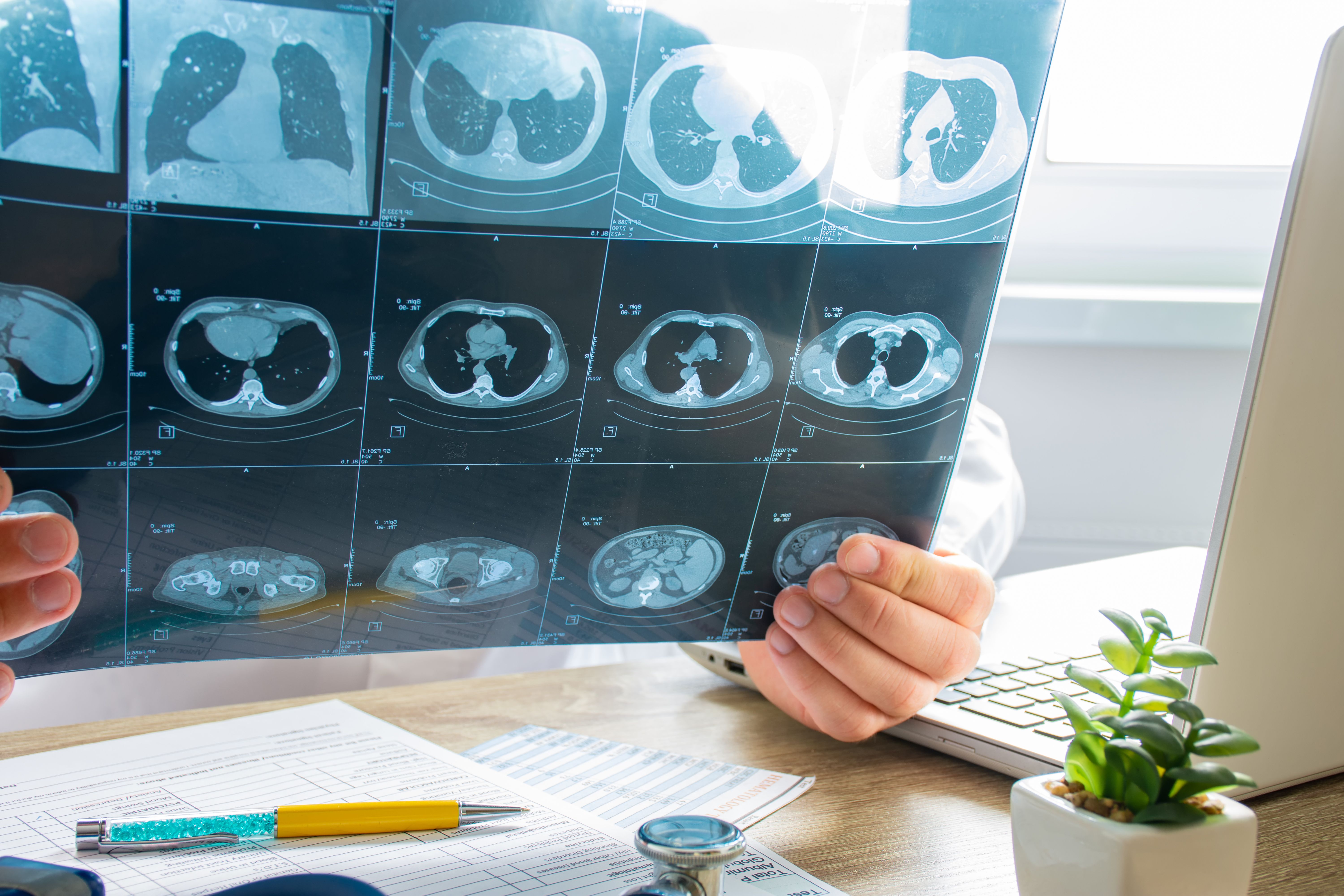
- Lung8, to measure lung volume
- Vascul8, to measure pulmonary vessel volume
- Fibr8, to measure fibrosis extent
- Air8, to measure airway volume
All AI models were trained on large imaging datasets and optimized to detect subtle structural changes in fibrotic lung tissue, according to the researchers.
They also observed strong correlations between Lung8-derived lung volumes and forced vital capacity (FVC) (r² = 0.83; P < .0001), as well as between normalized fibrosis and percent predicted FVC (r² = 0.52; P < .0001). This shows that, when properly trained, deep learning models can give doctors reliable, noninvasive snapshots of lung health that reflect real changes in breathing ability. These tools could eventually help track how a patient is responding to treatment without needing repeated invasive tests.
ENV-101 Preserved Lung Volume, Reduced Fibrosis
At 12 weeks, patients treated with ENV-101 showed statistically significant increases in lung volume compared with those receiving placebo, increasing by 142.28 mL with treatment and dropping by 113.07 mL with placebo (P = .014), corresponding with improvements in percent predicted FVC (P = .03).
The treatment arm also demonstrated a significant reduction in pulmonary vascular volume compared with placebo (–0.25% vs 0.07%; P = .0007) and a numerical trend toward fibrosis reduction (–1.32% vs 1.32%; P = .063). Patients receiving ENV-101 had less blood vessel swelling or congestion in their lungs and showed early signs of reduced lung scarring compared with those who got placebo. This suggests the drug may help slow or reverse lung damage in IPF. If confirmed in larger studies, these changes could mean better lung function and quality of life for patients.
Effect sizes were substantial across key metrics: 0.78 for percent predicted FVC, 0.87 for lung volume, and –1.28 for pulmonary vascular volume. These changes suggest ENV-101 may halt or reverse fibrotic progression in a subset of patients over a short treatment window.
“These results indicate that in IPF, deep learning–based quantification of lung volume and pulmonary vascular changes may provide additional insights that corroborate physiological improvement in lung function,” the researchers said.
ENV-101 for IPF
Although limited by sample size and short duration, this analysis underscores the potential of deep learning to complement traditional physiologic and radiologic endpoints in IPF trials. Back at ATS 2024, John Hood, PhD, cofounder and CEO of Endeavor BioMedicines, explained the potential advantages of ENV-101 over available IPF treatments pirfenidone and nintedanib.4 According to Hood, these 2 approved drugs still have tolerability issues like gastrointestinal toxicity and lack strong efficacy at slowing IPF progression, while ENV-101 is actually improving lung function and how patients feel.
“The biggest unmet medical need is we just don't have a therapy that makes patients better,” Hood told The American Journal of Managed Care® (AJMC®). “Ultimately, that's what we're trying to do at Endeavor. We have a drug that, at least to date, is showing evidence that patients get better.”
Hood also told AJMC only about 20% of patients with IPF are on standard of care with a high discontinuation rate.
“As far as what the health care system can do, they're somewhat limited because they simply don't have the tools in the toolbox yet,” he said. “We're trying to make it much, much easier for the health care system by giving them something that works.”
References
- Walsh SL, Difrancesco A, Hood J. Deep learning-based disease severity biomarkers on CT; posthoc analysis in a phase 2a placebo-controlled study of ENV-101 in subjects with idiopathic pulmonary fibrosis. Presented at: ATS 2025 International Conference; May 20, 2025; San Francisco, CA. https://www.atsjournals.org/doi/abs/10.1164/ajrccm.2025.211.Abstracts.A5339
- Zolak JS, de Andrade JA. Idiopathic pulmonary fibrosis. Immunol Allergy Clin North Am. 2012;32(4):473-485. doi:10.1016/j.iac.2012.08.006
- A study evaluating the safety and efficacy of ENV-101 in subjects with idiopathic pulmonary fibrosis (IPF). ClinicalTrials.gov. Updated December 2, 2024. Accessed June 25, 2025. https://clinicaltrials.gov/study/NCT04968574
- Klein HE, Santoro C. Dr John Hood highlights advantages of IPF hedgehog inhibitor ENV-101. AJMC. June 13, 2024. Accessed June 25, 2025. https://www.ajmc.com/view/dr-john-hood-highlights-advantages-of-ipf-hedgehog-inhibitor-env-101
