- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Advancing Care for Idiopathic Pulmonary Fibrosis (IPF): Evaluating Current Treatments and Emerging Therapies for Improved Patient Outcomes
What Is IPF?
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease characterized by the formation of fibrous tissue in the interstitium of the lungs.1 Though the exact cause remains unclear, current knowledge suggests that the pathogenesis of IPF involves repeated or chronic injury to the lung epithelium, which triggers the activation and differentiation of fibroblasts and myofibroblasts.2 The persistent myofibroblast activity leads to excessive extracellular matrix deposition and abnormal lung repair processes, resulting in formation of scar tissue, distortion of alveolar architecture, and ultimately, permanent loss of lung function.2 IPF presents as a progressive worsening of dyspnea as lung function deteriorates, leading to a poor prognosis.3 The median survival for individuals with IPF is an estimated 3 to 5 years after diagnosis.4 Respiratory hospitalizations are associated with a significantly reduced survival, with only about 50% of patients surviving 2 years following their first respiratory-related hospitalization.5
Impact of Disease
Population Impact
IPF impacts approximately 3 million people globally and 130,000 individuals in the US.6 The disease predominantly affects older adults, particularly those aged 60 to 70, with a higher prevalence among men.1,7 Although the exact etiology of IPF is unknown, risk factors include history of smoking, gastroesophageal reflux disease (GERD), chronic viral infections, hepatitis, and family history of interstitial lung disease (ILD).1 Several comorbidities are also found to be associated with IPF, such as pulmonary hypertension, pulmonary embolism, lung cancer, coronary artery disease, and obstructive sleep apnea.7 These comorbid conditions often arise well before an IPF diagnosis is made and contribute significantly to the clinical impact.7
Economic Impact
Between June 2014 and April 2016 in the US, IPF-PRO Registry, a prospective, observational, multicenter study, enrolled 300 participants with IPF to assess hospital-related resource use and costs, expressed in 2016 US dollars.7 The study reported 34% of patients having a hospital encounter during the first year of follow-up with a total of 158 hospital encounters.7 Of the patients who were hospitalized, 71% began their admissions in the emergency department; 69% of those were subsequently admitted to inpatient care, and 19% of the patients hospitalized were treated in the intensive care unit.7 Among patients who received a lung transplant, their hospital stays were significantly longer compared with those who did not undergo the procedure. Specifically, these patients had more than 4 times the duration of hospital stay (17.9 vs 4.0 days), nearly 15 times the length of ICU stay (7.3 vs 0.5 days), and approximately 20 times higher cost per hospital stay ($254,417 vs $13,795).7
Diagnostic Challenges
IPF is a diagnosis of exclusion, which may result in delays in both diagnosis and treatment initiation.5 Before IPF can be considered, other causes of ILD must be ruled out, leading to many patients initially being diagnosed with conditions such as heart failure or chronic obstructive pulmonary disease (COPD).8 On average, the time from initial respiratory-related hospitalization to an IPF diagnosis is 2.2 years.5 According to American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, Latin American Thoracic Society (ATS/ERS/JRS/ALAT) 2018 guidelines, adults with newly detected ILD of unknown origin should be assessed for IPF if they exhibit unexplained bilateral fibrosis on chest radiographs or computed tomography (CT) scans, bibasilar inspiratory crackles, and are older than 60 years.1 Early diagnosis of IPF is crucial, as a delay in initiating care is associated with a higher risk of death in IPF, regardless of disease severity.9 The hallmark of IPF is the presence of usual interstitial pneumonia patterns, which are best identified through high-resolution CT (HRCT) scans.1 HRCT is the gold standard for chest evaluation and is essential for accurate diagnosis of IPF.1
Disease Management
Guideline Recommendations in Managing IPF
The ATS/ERS/JRS/ALAT 2022 treatment guidelines for IPF include both pharmacologic and nonpharmacologic approaches. Antifibrotics (nintedanib and pirfenidone) are recommended along with nonpharmacologic options such as oxygen supplementation for hypoxemic patients and pulmonary rehabilitation.3 The ATS/ERS/JRS/ALAT guidelines also advise that patients with IPF should be thoroughly evaluated and treated for comorbidities, including pulmonary hypertension and GERD.3 In addition, involving patients in palliative care can be beneficial for managing symptoms.3
Obstacles in Managing IPF
Despite the development of IPF treatments, results of studies based on US registry data show that just 58% to 70% of patients with IPF are being treated with antifibrotic medications.10 This low rate of treatment is largely due to a “watch and wait” approach that physicians commonly practice for patients with mild to moderate disease.10 Additional challenges in IPF treatment include significant guideline variation in the use of antifibrotics in mild to moderate disease between countries.10 Up until 2023, the UK guidelines just recommended antifibrotic medications in patients with a forced vital capacity (FVC) between 50% and 80% despite knowing there were beneficial effects seen at higher FVC values.10,11 The greatest predictors of mortality in IPF are acute exacerbations of IPF (AE-IPF) and FVC decline greater than 15%.12
Unmet Needs in IPF Treatment
Whereas nintedanib and pirfenidone are effective in slowing down FVC decline, a 2024 systematic review and meta-analysis looked at real-world data across 74 studies with 23,119 participants and found higher rates of AE-IPF and mortality for both drugs in real-world settings compared with clinical trials.13 During clinical trials, these medications were also associated with adverse effects, particularly gastrointestinal issues and skin rashes, which were major factors in patient discontinuation.10,14,15 These discontinuation rates are also reflected in real-world data, which indicate that 10% to 20% of patients stop treatment due to adverse effects.10 High rates of AE-IPF, mortality, and adverse effect-related treatment discontinuation highlight the critical need for new antifibrotic therapies that improve disease prognosis and treatment tolerability, enabling better long-term adherence and improved patient outcomes in IPF management.
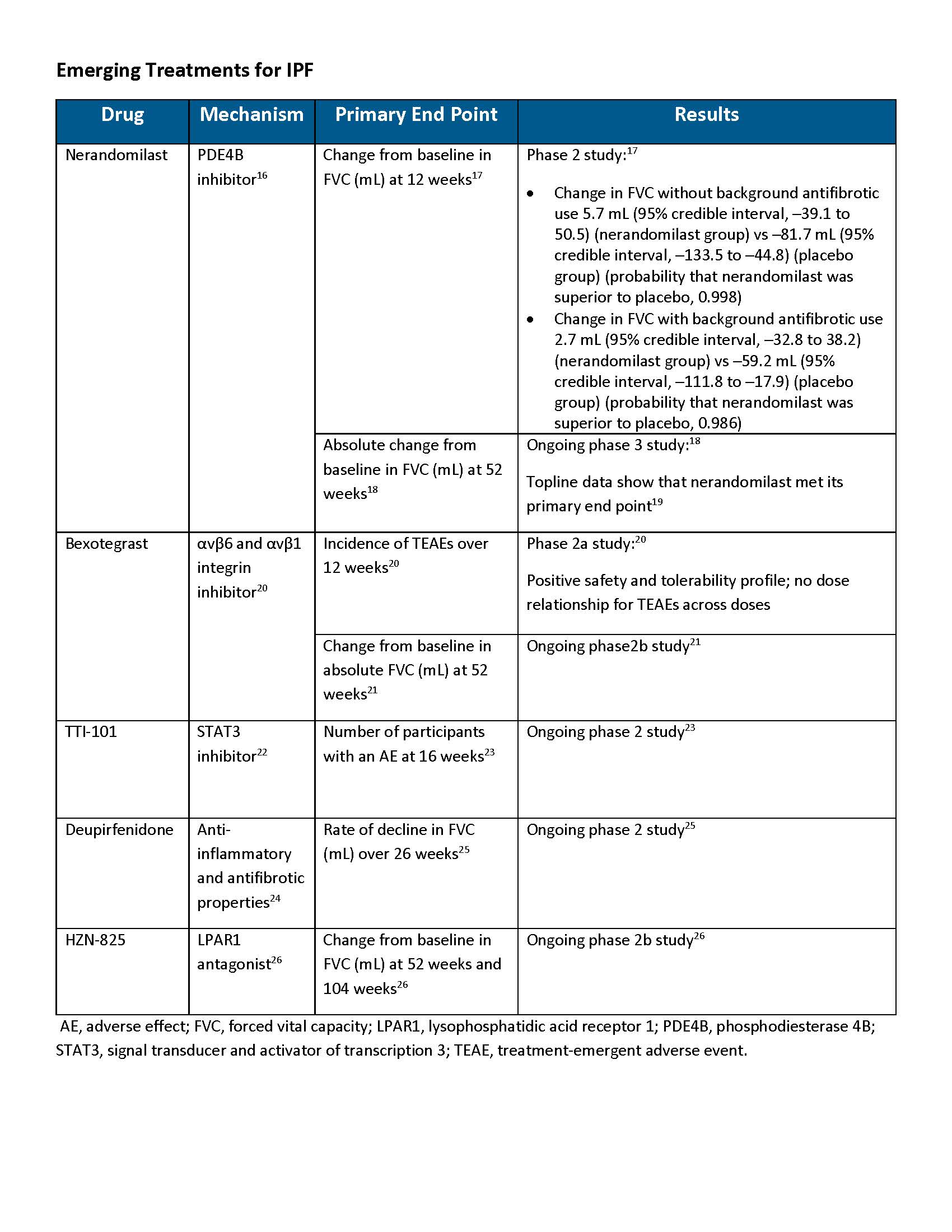
Potential for Phosphodiesterase 4 (PDE4) Inhibitors in IPF Treatment
PDE4 inhibitors are already being used for COPD to reduce exacerbations; however, there are none yet approved for IPF.27,28 PDE4 enzymes mediate the breakdown of cyclic adenosine monophosphate (cAMP) and are highly expressed in cells that regulate immune inflammatory responses and tissue remodeling.16,29 Inhibiting PDE4 results in elevated levels of cAMP, thereby producing its antifibrotic effects.29 PDE4 inhibition has been identified as a potential mechanism for the treatment of pulmonary fibrosis; however, the clinical use is often limited by dose-dependent adverse effects, particularly nausea and vomiting.16,30,31 There are 4 PDE4 subtypes (PDE4A, B, C, and D) with varied functions and tissue expression throughout the body. Vomiting is thought to be associated with the inhibition of the PDE4D subtype.31,32 Preferential inhibition of PDE4B is being investigated for the treatment of IPF as it may retain its anti-inflammatory and antifibrotic properties while avoiding certain use-limiting adverse effects.16,31
Conclusion
IPF is a progressive and fatal lung disease characterized by the scarring of lung tissue, leading to worsening respiratory function and significant morbidity. Its impact on patients is profound, with high health care costs, poor prognosis, and limited treatment options. Despite these challenges, recent clinical trial data offer hope, showcasing emerging therapies that may slow disease progression and improve patient outcomes. These advancements highlight the potential for new, more effective treatments, reinforcing the need for continued research and integration of novel therapies to address the unmet needs of patients with IPF and reduce the disease’s burden on health care systems.
REFERENCES
- Raghu G, Remy-Jardin M, Myers JL, et al; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
- Mei Q, Liu Z, Zuo H, Yang Z, Qu J. Idiopathic pulmonary fibrosis: an update on pathogenesis. Front Pharmacol. 2022;12:797292. doi:10.3389/fphar.2021.797292
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm/202202-0399ST
- Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140(1):221-229. doi:10.1378/chest.10-2572
- Herberts MB, Teague TT, Thao V, et al. Idiopathic pulmonary fibrosis in the United States: time to diagnosis and treatment. BMC Pulm Med. 2023;23(1):281. doi:10.1186/s12890-023-02565-7
- Martinez, FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi:10.1038/nrdp.2017.74
- Fan Y, Bender SD, Conoscenti CS, et al; IPF-PRO Registry Investigators. Hospital-based resource use and costs among patients with idiopathic pulmonary fibrosis enrolled in the Idiopathic Pulmonary Fibrosis Prospective Outcomes (IPF-PRO) Registry. Chest. 2020;157(6):1522-1530. doi:10.1016/j.chest.2019.12.041
- Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811-1823. doi:10.1056/NEJMra1705751
- Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842-847. doi:10.1164/rccm.201104-0668OC
- Alsomli H, Palmer E, Aujayeb A, Funston W. Early diagnosis and treatment of idiopathic pulmonary fibrosis: a narrative review. Pulm Ther. 2023;9(2):177-193. doi:10.1007/s41030-023-00216-0
- Idiopathic pulmonary fibrosis in adults: diagnosis and management. National Institute for Health and Care Excellence. Updated May 23, 2017. Accessed September 20, 2024. https://www.nice.org.uk/guidance/CG163
- Paterniti MO, Bi Y, Rekić D, Wang Y, Karimi-Shah BA, Chowdhury BA. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2017;14(9):1395-1402. doi:10.1513/AnnalsATS.201606-458OC
- Kou M, Jiao Y, Li Z, et al. Real-world safety and effectiveness of pirfenidone and nintedanib in the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2024;80(10):1445-1460. doi:10.1007/s00228-024-03720-7
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22)2083-2092. doi:10.1056/NEJMoa1402582
- Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082. doi:10.1056/NEJMoa1402584
- Richeldi L, Azuma A, Cottin V, et al. Design of a phase III, double-blind, randomised, placebo-controlled trial of BI 1015550 in patients with idiopathic pulmonary fibrosis (FIBRONEER-IPF). BMJ Open Respir Res. 2023;10(1):e001563. doi:10.1136/bmjresp-2022-001563
- Richeldi L, Azuma A, Cottin V, et al; 1305-0013 Trial Investigators. Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N Engl J Med. 2022;386(23):2178-2187. doi:10.1056/NEJMoa2201737
- A study to find out whether BI 1015550 improves lung function in people with idiopathic pulmonary fibrosis (IPF). ClinicalTrials.gov. Updated August 29, 2024. Accessed September 20, 2024. https://clinicaltrials.gov/study/NCT05321069
- Boehringer’s nerandomilast meets primary endpoint in pivotal phase-III FIBRONEER-IPF study. Boehringer Ingelheim. September 16, 2024. Accessed September 23, 2024. https://www.boehringer-ingelheim.com/human-health/lung-diseases/pulmonary-fibrosis/nerandomilast-primary-endpoint-phase-3-fibroneer-ipf
- Lancaster L, Cottin V, Ramaswamy M, et al; PLN-74809-IPF-202 Trial Investigators. Bexotegrast in patients with idiopathic pulmonary fibrosis: the INTEGRIS-IPF clinical trial. Am J Respir Crit Care Med. 2024;210(4):424-434. doi:10.1164/rccm202403-0636OC
- Randomized, double-blind study of efficacy and safety of bexotegrast (PLN-74809) for idiopathic pulmonary fibrosis. ClinicalTrials.gov. Updated September 4, 2024. Accessed September 20, 2024. https://clinicaltrials.gov/study/NCT06097260?term=BEACON%20IPF&rank=1
- Tsimberidou AM, Vining DV, Arora SP, et al. Phase 1 trial evaluating TTI-101, a first-in-class, orally bioavailable, small molecule, inhibitor of STAT3, in patients with advanced solid tumors. J Clin Oncol. 2023;41(16): Abstract 3018. doi:10.1200/JCO.2023.41.16
- Study of TTI-101 in participants with idiopathic pulmonary fibrosis. ClinicalTrials.gov. Updated August 22, 2024. Accessed September 20, 2024. https://clinicaltrials.gov/study/NCT05671835?cond=IPF&intr=TTI-101%20&rank=1
- Chen MC, Korth CC, Harnett MD, Elenko E, Lickliter JD. A randomized phase 1 evaluation of deupirfenidone, a novel deuterium-containing drug candidate for interstitial lung disease and other inflammatory and fibrotic diseases. Clin Pharmacol Drug Dev. 2022;11(2):220-234. doi:10.1002/cpdd.1040
- LYT-100 in patients with idiopathic pulmonary fibrosis (IPF (ELEVATE). ClinicalTrials.gov. Updated May 7, 2024. Accessed September 20, 2024. https://clinicaltrials.gov/study/NCT05321420?cond=IPF&intr=Deupirfenidone%20(LYT-100)%20&rank=1
- A multicenter trial to evaluate the efficacy, safety and tolerability of HZN-825 in subjects with idiopathic pulmonary fibrosis. ClinicalTrials.gov. Updated August 9, 2024. Accessed September 20, 2024. https://clinicaltrials.gov/study/NCT05032066?cond=IPF&intr=HZN-825&rank=1
- Daliresp. Prescribing information. AstraZeneca Pharmaceuticals; 2020. Accessed September 25, 2024. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/704932ce-e104-4c6f-840a-575170971344/704932ce-e104-4c6f-840a-575170971344_viewable_rendition__v.pdf
- Martinez FJ, Calverley PMA, Goehring U, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857-866. doi:10.1016/S0140-6736(14)62410-7
- Yang X, Xu Z, Hu S, Shen J. Perspectives of PDE inhibitor on treating idiopathic pulmonary fibrosis. Front Pharmacol. 2023;14:1111393. Doi:10.3389/fphar.2023.1111393
- Kolb M, Crestani B, Maher TM. Phosphodiesterase 4B inhibition: a potential novel strategy for treating pulmonary fibrosis. Eur Respir Rev. 2023;32(167):220206. doi:10.1183/16000617.0206-2022
- Herrmann FE, Hesslinger C, Wollin L, Nickolaus P. BI 1015550 is a PDE4B inhibitor and a clinical drug candidate for the oral treatment of idiopathic pulmonary fibrosis. Front Pharmacol. 2022;13:838449. doi:10.3389/fphar.2022.838449
- Azevedo MF, Faucz FR, Bimpaki E, et al. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev. 2014;35(2):195-233. doi:10.1210/er.2013-1053
