- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Ziresovir Reduces Bronchiolitis in Infants Hospitalized With RSV
Ziresovir reduces respiratory syncytial virus (RSV) symptoms and viral load in infants, offering hope for an effective treatment.
A phase 3 clinical trial conducted in China evaluated the efficacy of ziresovir in infants hospitalized with respiratory syncytial virus (RSV), finding ziresovir significantly improved clinical outcomes, reducing the severity of bronchiolitis symptoms and viral load compared with placebo.1
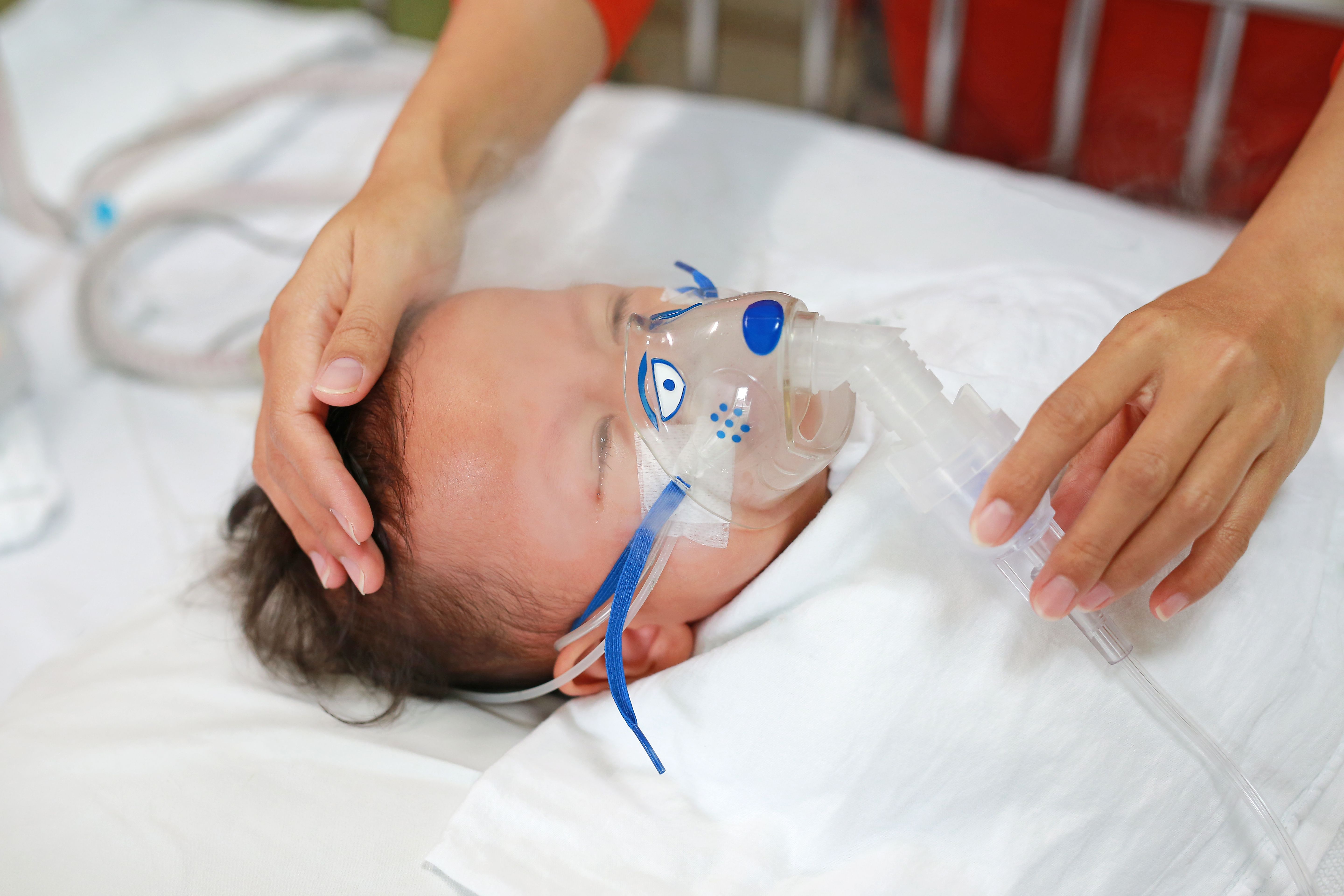
The multicenter, double-blind, randomized, placebo-controlled trial is published in The New England Journal of Medicine.
“RSV infection has long been a serious medical challenge in pediatric health care,” said Xin Ni, MD, PhD, a leading principal investigator at Beijing Children's Hospital, Capital Medical University, in a statement.2 "This virus not only poses a threat to children's health, but also places a heavy burden on our health care system. For a long time, we have been searching for a safe and effective treatment.”
Conducted across various sites in China, infants aged 1 to 24 months hospitalized with RSV were randomly assigned 2:1 to receive either ziresovir, at doses ranging from 10 to 40 mg, or placebo, administered twice daily over a 5-day period.1
The primary outcome was the change in the Wang bronchiolitis clinical score from baseline to day 3, with higher scores indicating more severe symptoms. Secondary outcomes included reductions in RSV viral load by day 5.
The trial's findings demonstrate that ziresovir significantly improved clinical outcomes in infants and young children hospitalized with RSV. In the intention-to-treat population of 244 participants, the reduction in the Wang bronchiolitis clinical score by day 3 was significantly greater in the ziresovir group compared with the placebo group (−3.4 points vs −2.7 points; P = .002).
Additionally, ziresovir led to a larger reduction in RSV viral load by day 5 (−2.5 vs −1.9 log10 copies/mL), indicating enhanced antiviral activity.
The drug's efficacy was consistent across prespecified subgroups, including participants with more severe bronchiolitis and those younger than 6 months. Adverse events were comparable between the ziresovir and placebo groups, with common events including diarrhea, elevated liver enzyme levels, and rash. Notably, 9% of participants in the ziresovir group developed resistance-associated mutations, but no significant safety concerns were identified overall. These results suggest that ziresovir effectively reduces RSV symptoms and viral load in infants with a favorable safety profile.
The researchers acknowledged some limitations to the study. First, the Wang bronchiolitis clinical score is not fully validated in studies of RSV infections. Second, because the study was conducted in China, it may not be applicable to other regions. Lastly, the researchers did not record the time to the discontinuation of supplemental oxygen or nebulizer use as potential clinical markers of improvement.
Despite these limitations, the researchers believe these findings suggest ziresovir could offer an effective therapeutic option for RSV in young children.
“Under the guidance of the regulatory agencies, we will continue to develop the drug in collaboration with the medical community” said Jim Wu, PhD, CEO of ArkBio, which is developing ziresovir, in a statement.2 “We are committed to the research and development of innovative medicines to safeguard the wellbeing and healthy growth of children."
References
1. Zhao S, Shang Y, Yin Y, et al. Ziresovir in hospitalized infants with respiratory syncytial virus infection. N Engl J Med. 2024;391(12):1096-1107. doi:10.1056/nejmoa2313551
2. ArkBio announces publication of phase 3 clinical trial results of ziresovir for the treatment of RSV infection in The New England Journal of Medicine. News release. ArkBio. September 26, 2024. Accessed September 27, 2024. https://www.prnewswire.com/apac/news-releases/arkbio-announces-publication-of-phase-3-clinical-trial-results-of-ziresovir-for-the-treatment-of-rsv-infection-in-the-new-england-journal-of-medicine-302259902.html.
The Breakdown: Breast Cancer Research Awareness Day
August 19th 2025Breast cancer is the second most common cancer among women and the second leading cause of cancer-related deaths among women in the US. In light of Breast Cancer Research Awareness Day, The American Journal of Managed Care® breaks down the most recent advancements in breast cancer prevention, screening, and therapies.
Listen
Politics vs Science: The Future of US Public Health
February 4th 2025On this episode of Managed Care Cast, we speak with Perry N. Halkitis, PhD, MS, MPH, dean of the Rutgers School of Public Health, on the public health implications of the US withdrawal from the World Health Organization and the role of public health leaders in advocating for science and health.
Listen
