- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Young Woman With Rare Cervical Cancer Achieves Remission After Nivolumab Before Chemoradiotherapy
A tumor board looked to studies in ovarian cancer for guidance on use of immunotherapy in a rare case of clear cell carcinoma of the cervix.
A recent case report highlights the challenges of treating a rare type of cervical cancer, while also showing how clinicians can glean insights from reports of treating related cancers.
Clear cell carcinoma | Image credit: Sunnybrook Health Sciences Center
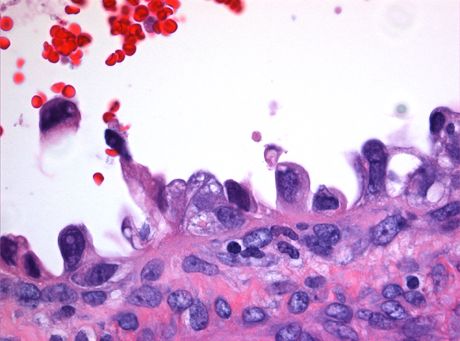
Writing in Gynecologic Oncology Reports, authors from the Geneva University Hospitals of Switzerland shared details of the case of a 38-year-old woman with a rare type of clear cell carcinoma of the cervix, which was discovered after she sought treatment following bleeding after intercourse. Although the lesion was diagnosed at an advanced stage, cancer had not spread to the pelvic wall or metastasized to distant sites.
The authors note that clear cell carcinoma “is a rare and very aggressive subset of cervical cancer, with poor outcome if diagnosed at advanced stage. There are few data available on the optimal management of this histotype, and treatment recommendations that include surgery and chemoradiotherapy, are essentially based on those for squamous cell carcinoma.”
A tumor board developed a recommendation to take advantage of a “window of opportunity” to give the patient a single injection of the PD-1 inhibitor nivolumab, followed by standard chemoradiotherapy. According to the authors, the board looked to studies that showed this type of clear cell carcinoma of the ovary was less sensitive to platinum-based chemotherapy than other types and may be radioresistant as well. (Prior to treatment, the patient was advised to undergo fertility preservation, the authors said.)
The woman experienced a complete remission, which persisted at 28 months of follow-up. However, as a result of immunotherapy treatment, she required lifelong thyroid hormone replacement; the authors stated this can occur in about 7% of such cases. Balanced against the patient’s age and her poor prognosis when the tumor was found, they wrote, “It is important to balance the expected benefits of a treatment against potential toxicities.”
A rare cancer. The authors wrote that little is known about the microenvironment of clear cell carcinoma of the cervix. Once again looking to a phase 1/2 study of PD-1 blockade in ovarian cancer, subgroup analyses suggested higher response rates in the clear cell carcinoma subtype.
“The patient is in complete remission 28 months after the end of treatment, which is encouraging given the high relapse rate and mortality of the disease. Multiplexed [immunohistochemistry] analysis of the tumor revealed an increased CD8/FoxP3 ratio, a potential biomarker associated with higher response to neoadjuvant immune checkpoint blockade in advanced stage cervical cancer,” they wrote.
Although a subsequent study in cervical cancer introduced a new standard of frontline care, there were no patients with clear cell carcinoma included. Thus, for the individual young woman in this case, the authors said, “It remains uncertain whether the complete remission is due to the single dose of immunotherapy prior to chemoradiotherapy.”
Reference
Courtes MG, Kountouri M, Wang W, et al. Window of opportunity with PD1 blockade before chemoradiotherapy for an advanced stage clear cell carcinoma of the cervix. Gynecol Oncol Rep. 2024;53:101304.
