- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Will the Lack of Long-Term Safety Data Hurt PCSK9 Repatha and Praluent Reimbursement?
As Amgen's Repatha (evolocumab) and Sanofi/Regeneron's Praluent (alirocumab) get closer to market, the safety question is one of the remaining pieces of the puzzle that unfortunately can't be solved immediately until real-world data starts to become available. However, as payers assess the risk-benefit of Repatha and Praluent vs the gold standard statins, there are questions that CAN be answered immediately.
Patrick Gleason, PharmD, director of health outcomes at Prime Therapeutics, LLC, recently told AIS’s Specialty Pharmacy News that insurers “will need to consider the lack of long term safety studies in a condition that is frequently treated for a lifetime” when debating where on formulary to place the PCSK9s. As Amgen’s Repatha (evolocumab) and Sanofi/Regeneron’s Praluent (alirocumab) get closer to market, the safety question is one of the remaining pieces of the puzzle that unfortunately can’t be solved immediately until real-world data starts to become available.
However, as payers assess the risk-benefit of Repatha and Praluent vs the gold standard statins, there are questions that CAN be answered immediately.
What Does the Real-World Data Show for Statins?
Although statins have been on the market for a long time, payers should not confuse market experience with a completely safe profile. And when performing comparative effectiveness research, healthcare decision makers should not rely on safety information from drug label inserts, pharmaceutical company-prepared dossiers, and clinical trial results alone. Such data are limited in scope by publication bias, commercial interests, disclosure delays and the relative homogeneity of clinical trial participants.
In order to understand the true safety profile of any medication, regardless of time on market, healthcare decision makers should look toward real-world data to identify ongoing safety concerns and more importantly, calculate real-world costs associated with those concerns.
Our proprietary algorithm, RxCost, calculates downstream medical costs associated with adverse events(AEs)/drug combinations. In this way payers can use an independent, standardized methodology to plug into budget impact models to truly understand the impact that medications can have on their patient population.
The table below shows the top 5 most costly serious AEs reported for the most widely used statins: Lipitor, Crestor, and Zocor. For a full RxCost comparison of the statins, including an overall RxCost per prescription, download the recent Drug Safety Monitor report, Safety analysis of PCSK-9 inhibitors: evolocumab and alirocumab vs. Lipitor.
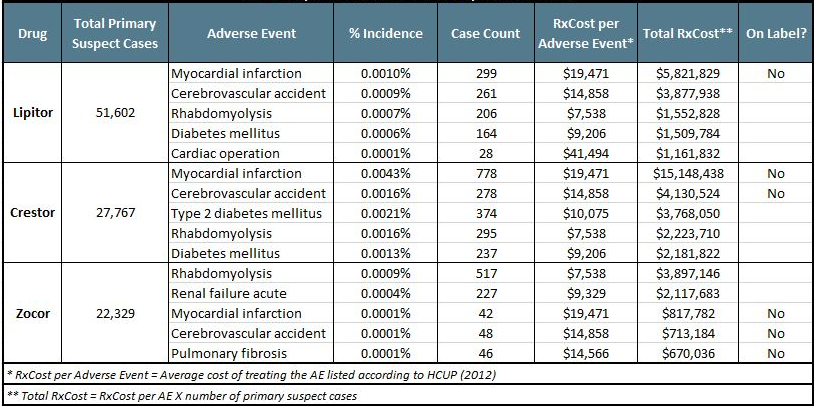
How Do Clinical Trial Data for Repatha and Praluent Compare With the Post Approval Experience With Statins?
A preliminary analysis, that can be seen in full by downloading the above mentioned report shows that the anti-PCSK9s have a similar safety profile to Lipitor, with additional risks reported during clinical trials for injection site AEs, antibody-related AEs, and neurocognitive AEs. Lipitor's label indicates AEs related to the hepatic system, toxic epidermal necrolysis, Steven-Johnsons Syndrome, and pancreatitis, which had not been reported for the anti-PCSK9s in their respective clinical trials. One noteworthy distinction between the 2 anti-PCSK9s was that the alirocumab clinical trial (ODYSSEY) showed an increased rate of ophthalmic events compared to placebo (2.9% vs 1.9%), which was not reported in the evolocumab trials (DESCARTES, OSLER-1 and 2).
The following table shows evolocumab’s AEs with corresponding reporting odds ratio (ROR) values and primary suspect case counts for Lipitor. To see alirocumab’s comparison chart, follow the link above to download the full report.

What Processes Will Be Put in Place to Monitor the PCSK9s Once They Do Come to Market?
With payers citing curbing rising drug costs and curbing rising medical (non-drug) costs as their biggest business challenges (EY Progressions 2014 Payer Survey), there is just too much at stake at the bottom line to wait for FDA action to identify potential safety issues. Managed care organizations must put in place processes for post-marketing surveillance at the organizational level; however, there are many challenges to designing and implementing an analytics program of this magnitude. Limited datasets, unstandardized analytics without widely accepted methodologies, and organizational misalignment all contribute to failed projects and missed opportunities.
An easier and more economically feasible way to stay ahead of potential safety concerns would be to implement an out-of-the box solution that provides an on-demand intuitive user interface designed for all organizational levels, containing independent and unbiased data and scientifically validated methodologies.
A system, such as the one provided by my organization, AdverseEvents Explorer would allow managed care organizations to stay ahead of any PCSK9 safety concerns and proactively assess the downstream medical costs from drug side effects as soon as real world data is available, that can improve patient safety and reduce overall costs.
Early Data Show Promising Outcomes as CAR T-Cell Therapy Expands Beyond Academic Centers
January 12th 2026Abstracts presented at ASH 2025 explore CAR T-cell therapy's effectiveness in newly authorized treatment centers, revealing promising outcomes for multiple myeloma patients and the prospect of expanded access.
Read More
