- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Updates and Evolving Treatment Strategies in Acute Myeloid Leukemia
To claim CE credit for this activity, please visit https://www.pharmacytimes.org/courses/updates-and-evolving-treatment-strategies-in-acute-myeloid-leukemia
Introduction
Acute myeloid leukemia (AML) is a malignant disease of the hematopoietic system caused by clonal proliferation of a myeloid progenitor cell. Although AML is the most common type of leukemia in adults, AML is a relatively rare disease, accounting for just 1% of new cancer diagnoses in the United States.1 The median age at diagnosis is 68 years, with patients 55 years or older comprising over 75% of newly diagnosed cases.1
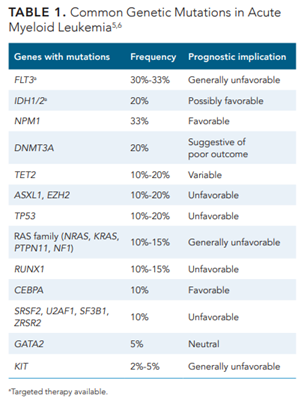
Irrespective of treatment modality, outcomes in older adults with AML are worse than young adults due to both disease-related and patient-specific factors. Patients older than 64 years have a 5-year relative survival rate of just 9.4%.1 Antecedent hematologic disorders (myelodysplastic syndrome [MDS] and myeloproliferative neoplasms) or secondary AML occur in 24% to 40% of older patients diagnosed with AML, portending difficult-to-treat disease.2 In addition, older patients with AML typically have higher numbers of chromosomal aberrations, including mutations in genes that confer resistance to many treatments.3 In particular, TP53-mutated AML is less likely to respond to conventional chemotherapy treatments; the overall survival (OS) at 2 years is less than 10%.4 Molecular abnormalities not only inform prognostic discussions, but also can be the basis for targeted therapies. See Table 15,6 for common genetic mutations in AML.
For older patients considered fit for intensive therapy, the standard-of-care treatment for newly diagnosed AML is a cytotoxic chemotherapy induction, 7 plus 3, comprising myeloablative doses of an anthracycline, daunorubicin or idarubicin, and continuous infusion cytarabine, or a liposomal formation of cytarabine and daunorubicin. Although successful in inducing remission in 40% to 60% of patients, induction chemotherapy carries a high risk of morbidity and mortality due to infection, bleeding, and other complications from pancytopenia.7-9 Anthracycline-containing induction chemotherapy also carries a risk of cardiotoxicity.10 Post-remission therapy, or consolidation, is recommended for patients who have achieved complete remission (CR) to prevent disease recurrence, as relapse risk is greater than 50% for adults with high-risk AML.11 Based on risk stratification, many patients will need to proceed to hematopoietic stem cell transplant (HSCT) for a definitive cure.8
Due to the high level of morbidity and mortality associated with intensive treatment, older patients were historically not considered candidates for cytotoxic induction chemotherapy. Outpatient treatments such as the hypomethylating agents azacitidine and decitabine have been used over the past 20 years based on data demonstrating survival advantages over best supportive care alone.12,13 Despite this evidence, registry studies indicate that as many as 50% of older patients diagnosed with AML do not receive any anti-leukemia therapy.14 In 2020, the American Society of Hematology published a consensus guideline to address the treatment of older adults (≥55 years) with newly diagnosed AML. The consensus panel’s meta-analysis demonstrated that anti-leukemia treatment has an OS advantage over best supportive care, with potential harms and burdens considered to be small (strong recommendation based on moderate certainty in the evidence of effects).3 As a larger percentage of elderly patients may be considered for therapy and/or HSCT, there is increased rationale for alternative therapies and incorporation of targeted agents, specifically designing regimens that are not as intensive as conventional induction chemotherapy.

Clinical Data for Alternative Induction Regimens
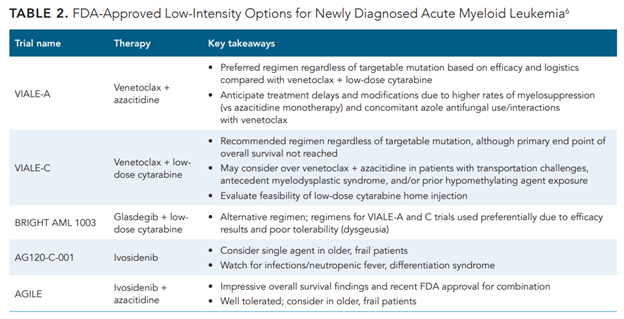
Treatment options for patients with newly diagnosed AML have grown exponentially in the past 5 years due to the approval of novel agents. The FDA has approved 5 lower-intensity regimens for patients who are treatment naïve and ineligible for intensive chemotherapy (Table 26). The National Comprehensive Cancer Network (NCCN) guidelines provide a framework for approaching patients 60 years or older who are not candidates for intensive therapy, including the use of FDA-approved therapies as well as agents and combinations with clinical benefit. Categories of therapy recommendations are stratified based on mutation status: AML without actionable mutation, isocitrate dehydrogenase (IDH) 1 or 2 mutation, or fms-like tyrosine kinase 3 (FLT3) mutation.15
Venetoclax Combinations
Across all 3 groups, the NCCN category 1 recommendation for preferred therapy is venetoclax plus azacitidine, based on the landmark trial VIALE-A that was published in 2020. VIALE-A was a multicenter, double-blind, placebo-controlled, randomized, phase 3 trial in patients with newly diagnosed AML who were ineligible for standard induction therapy per Ferrari criteria. Of note, patients were ineligible if they had previously received a hypomethylating agent. The study group received intravenous (IV) azacitidine days 1 to 7 and oral venetoclax days 1 to 28 of a 28-day cycle; the control group received IV azacitidine days 1 to 7 and oral placebo days 1 to 28 of a 28-day cycle. The primary end point was median OS; secondary end points included rates of CR, red cell and platelet transfusion independence, measurable residual disease (MRD), as well as OS in molecular and cytogenetic subgroups. Median OS was 14.7 months in patients treated with venetoclax plus azacitidine versus 9.6 months for patients treated with azacitidine alone (HR, 0.66; P <.001). CR was achieved in 36.7% and 17.9% of patients, respectively (P <.001); transfusion independence was higher in the venetoclax plus azacitidine cohort. When assessing response in patients with genetic mutations, high response rates occurred for those with FLT3, IDH1, and IDH2 mutations. Rates of myelosuppressive adverse effects (AEs) differed between the groups. The venetoclax plus azacitidine group experienced higher rates of myelosuppression, including neutropenia (42% vs 28%), febrile neutropenia (42% vs 19%), and thrombocytopenia (45% vs 38%), all of which were reported at grade 3 or higher.16
Dose interruptions, including reductions in treatment duration and delays between cycles, occurred in 53% of patients receiving venetoclax plus azacitidine versus 28% of patients receiving azacitidine alone, highlighting potential complexities of managing treatment outside of a clinical trial. Nearly half of the patients randomized had at least 2 reasons for ineligibility for intensive treatment, yet treatment discontinuation was similar between the 2 groups (24% vs 20%) despite higher rates of myelosuppression and dose delay in the venetoclax plus azacitidine group, suggesting this regimen is tolerable for this patient population if recommended dosing parameters are followed.16
Management of venetoclax in combination with chemotherapy can be challenging for several reasons. As described previously, many patients will require dose reductions, interruptions, and cycle delays that can lead to confusion both for the patient and the healthcare team; multidisciplinary care including a pharmacist is vital to ensure patients are receiving appropriate prescriptions, instructions, and follow-up assessments. Venetoclax combination therapy frequently warrants antimicrobial prophylaxis due to high rates of neutropenia; however, many anti-mold medications (voriconazole, posaconazole, isavuconazonium sulfate) interact with venetoclax via the CYP3A4 pathway, requiring venetoclax dose adjustment. Although the venetoclax prescribing information provides comprehensive dose adjustment recommendations, very little data exist reporting outcomes for patients who received dose adjustments due to drug-drug interactions, raising concerns that these dose reductions may theoretically decrease the efficacy of treatment.17,18
An alternative to the VIALE-A regimen is venetoclax plus decitabine, another hypomethylating agent. This combination was studied in the phase 1b/2 dose escalation and expansion phases of VIALE-A and showed similar safety signals as venetoclax plus azacitidine. Patients received venetoclax daily days 1 to 28 and IV decitabine days 1 to 5 of each 28-day cycle. Doses of venetoclax ranged from 400 mg to 1200 mg daily. In the phase 1b study, median OS for venetoclax plus decitabine was 16.2 months, and the CR/CR with incomplete blood count recovery (CR/CRi) rate was 74%, with a median duration of CR/CRi of 15 months. Although not studied in a randomized controlled trial, venetoclax plus decitabine was FDA approved based on the rate of CR and CR duration in these phase 1/2 studies.19,20
Venetoclax plus low-dose cytarabine (LDAC) is a recommended option for this patient population, regardless of targetable mutation status, based on the results of VIALE-C, a randomized, double-blind, placebo-controlled, phase 3 study. Eligible patients included those with previously untreated AML who were 75 years or older or were ineligible for intensive chemotherapy due to reduced performance status, organ dysfunction, and/or any comorbidity judged to be incompatible with intensive chemotherapy. Patients with secondary AML from MDS were included and could have received previous therapy with a hypomethylating agent. The study group received subcutaneous cytarabine once daily on days 1 to 10 and oral venetoclax days 1 to 28 of a 28-day cycle; the control group received the same cytarabine therapy and oral placebo days 1 to 28 of a 28-day cycle. The primary objective was OS; secondary end points included rates of CR, CR with partial hematologic recovery (CRh), and rates of transfusion independence. Median OS was 7.2 months in patients receiving venetoclax plus LDAC versus 4.1 months in the control group (HR, 0.75; P = .11); thus, the study group failed to meet the specified primary end point. After an additional 6 months, the median OS was 8.4 months versus 4.1 months, respectively (HR, 0.70; P = .04). Rates of CR were 27% in the venetoclax plus LDAC group compared with 7% in the placebo group (P <.001); CR plus CRh was 47% versus 15%, respectively (P <.001). Transfusion independence rates were higher for patients treated with venetoclax plus LDAC (37% vs 16%; P = .002). Rates of myelosuppressive AEs differed between the groups. The venetoclax plus LDAC group experienced higher rates of neutropenia (46% vs 16%), febrile neutropenia (32% vs 29%), and thrombocytopenia (45% vs 37%), all of which were reported at grade 3 or higher. Nonhematologic events included nausea (42% vs 31%), hypokalemia (28% vs 22%), diarrhea (28% vs 16%), and constipation (18% vs 31%), but few were high grade.21 Although the trial did not meet the primary end point of OS improvement, venetoclax plus LDAC is a reasonable alternative to venetoclax plus azacitidine; LDAC is usually administered via subcutaneous injection in a patient’s home while hypomethylating agents must be administered in a healthcare facility, so this regimen may provide an alternative for patients who have difficulty traveling to healthcare visits or wish to spend more time at home for potential improved quality of life (QOL). Unfortunately, logistical barriers occur commonly with LDAC, including lack of insurance approval for the medication and/or home health services, and patients may have hesitations about self-administration of chemotherapy.
Hypomethylating Agents
Single-agent decitabine or azacitidine was a commonly used treatment before the publication of VIALE-A, as several noncomparative trials showed benefit in patients with AML unfit for induction chemotherapy. Two phase 3 studies followed up, and the subgroup analysis demonstrated better outcomes versus best supportive care in patients with 20% to 30% AML blasts.22 AZA-AML-001 compared azacitidine with 3 conventional care regimens (LDAC, intensive chemotherapy, and supportive care alone), and azacitidine demonstrated longer median OS (10.4 vs 6.5 months).13 Decitabine compared with the investigator’s choice (most commonly LDAC) was associated with a survival advantage in a post hoc analysis.12
Hypomethylating agents have poor bioavailability due to inactivation via gastrointestinal cytidine deaminase. Cedazuridine, a cytidine deaminase inhibitor, is combined with decitabine; this combination oral formulation is FDA approved for treatment of MDS.23 Recently, the ASCERTAIN-AML randomized, phase 3 trial compared patients with AML receiving oral decitabine/cedazuridine with IV decitabine to determine area-under-the-curve equivalence. The primary end point was achieved, demonstrating exposure bioequivalence, so decitabine/cedazuridine will likely receive an approval for use in patients with AML in the near future.24
Isocitrate Dehydrogenase Mutation Inhibitors
For patients with an IDH mutation, the NCCN guidelines preferred treatments include venetoclax plus a hypomethylating agent (category 1 with azacitidine) or treatment with an agent targeting the IDH mutation. In 2019, ivosidenib was the first IDH-targeted agent FDA approved for elderly patients with newly diagnosed AML based on the phase 1 study AG120-C-001. The study was an open-label, single-arm, dose-escalation and dose-expansion trial that enrolled patients 75 years or older or with comorbidities preventing the use of intensive induction chemotherapy. All patients had a documented IDH1-mutated hematologic cancer. Patients received single-agent ivosidenib daily until progression, toxicity, or HSCT. The primary objectives were to assess safety, maximum tolerated dose, and clinical activity in a cohort of patients with relapsed or refractory AML.25 A subset of patients with newly diagnosed AML achieved a CR rate of 30.3% (95% CI, 15.6-48.7), with an additional 42.4% achieving CR or CRh (95% CI, 25.5-60.8). Median OS was 12.6 months (95% CI, 4.5-25.7 months). Of note, 47% of patients had prior exposure to a hypomethylating agent; the CR plus CRh rate of that pretreated group was 26.7% (95% CI, 7.8-55.1). The most common treatment-related AEs were diarrhea (26%), fatigue (21%), nausea (18%), and decreased appetite (18%). AEs of special interest included treatment-related leukocytosis (6%), QT prolongation (18%) requiring dose interruption in 4 patients (12%), and treatment-related differentiation syndrome (18%) leading to dose interruption in 3 patients (9%).26
Although not included as a recommended regimen in NCCN guidelines as of June 2022, a newly FDA-approved combination regimen of ivosidenib plus azacitidine may be added to guidelines soon due to results of the phase 3 AGILE trial. This double-blind, randomized, placebo-controlled trial evaluated the safety and efficacy of ivosidenib and azacitidine versus placebo and azacitidine in patients with newly diagnosed, IDH1-mutated AML who were 75 years or older or considered ineligible for intensive induction chemotherapy. Median OS was 24.0 months in the ivosidenib plus azacitidine group and 7.9 months in the placebo plus azacitidine arm (HR, 0.44; 95% CI, 0.27-0.73; P = .0010). CR occurred in 47% of patients in the study arm versus 15% of patients in the placebo arm (P <.001). Overall rates of hematologic AEs were higher in the ivosidenib plus azacitidine group versus the placebo group (77% vs 66%); however, the percentage of patients with infections was lower in the study arm (25% vs 49%). The rate of differentiation syndrome was 14% with ivosidenib plus azacitidine and 8% with placebo plus azacitidine; median time to onset was 19.5 days. AEs led to higher rates of therapy interruption in the study arm (52%) versus the placebo arm (38%), most of which were driven by neutropenia or febrile neutropenia events.27
Enasidenib is an oral agent targeting the IDH2 mutation; although not FDA approved for newly diagnosed AML, NCCN guidelines recommend enasidenib as a preferred agent for older patients with IDH2-mutated AML who are not candidates for intensive therapy. A phase 1/2, open-label, dose-escalation and expansion trial enrolled patients with IDH2-mutated advanced myeloid malignancies; cohorts included older patients with relapsed/refractory AML, those with newly diagnosed AML ineligible for induction chemotherapy, younger patients with relapsed/refractory AML without HSCT, and any patient relapsed after HSCT.28 A subset of 39 patients with newly diagnosed AML achieved an overall response rate of 30.8%, including 7 patients with a CR (18%). Estimated median OS at data cutoff was 11.3 months (95% CI, 5.7-15.1). The most common treatment-related AEs were nausea (23%), fatigue (18%), decreased appetite (18%), rash (18%), and anemia (15%). AEs of special interest included treatment-related differentiation syndrome (DS) (13%) leading to dose interruption in 4 patients (10%) and indirect hyperbilirubinemia (31%).29 As enasidenib inhibits the UGT1A1 enzyme that glucuronidates bilirubin, isolated indirect hyperbilirubinemia does not indicate liver toxicity but is similar to the effects seen by congenital UGT1A1 deficiency (eg, Gilbert syndrome).30
As IDH inhibitors are associated with myeloid maturation and differentiation of leukemia cells, DS may occur, causing pulmonary infiltrates, dyspnea, weight gain, fever, acute renal failure, and/or hypotension; DS can be life-threatening or fatal, especially if not treated urgently with high-dose dexamethasone 10 mg IV every 12 hours (or an equivalent dose of alternative corticosteroid) and hemodynamic monitoring.31,32 Patients most commonly experience DS symptoms within the first 3 weeks of therapy; however, isolated cases have occurred months after starting treatment with an IDH inhibitor. Pharmacists are especially well poised to help educate patients on the signs and symptoms of DS, as most patients will receive the agents solely in the outpatient setting and may not have as close follow-up as patients routinely visiting infusion centers to receive IV therapies.33
Hedgehog Inhibitors
The BRIGHT AML 1003 trial evaluated a first-in-class oral small-molecule inhibitor of the Hedgehog signaling pathway, glasdegib, combined with LDAC. The trial was a phase 2, randomized open-label, multicenter study evaluating safety and efficacy in patients with AML or high-risk MDS who were previously untreated and not eligible for intensive chemotherapy. Although the trial demonstrated improved OS of the study arm compared with LDAC alone (8.8 months vs 4.9 months; HR, 0.51; 80% CI, 0.39-0.67; P = .0004), the AE profile for glasdegib plus LDAC was significant, causing 47 patients (56%) to temporarily discontinue glasdegib and/or LDAC and 22 (26%) to require a dose reduction due to AEs. Known AEs of the Hedgehog pathway inhibitors occurred in this trial, including muscle spasms (17.9%, grade ≥3: 4.8%), dysgeusia (25%), decreased appetite (29.8%), and alopecia (6%). Because these AEs affect patient QOL, glasdegib plus LDAC is not used as frequently in practice given other alternatives.34
The approval of ivosidenib plus azacitidine hints at the future of treatment of older patients with AML; many combination therapies are currently being studied, including combinations of targeted agents, hypomethylating agents, and venetoclax to determine both safety and efficacy. What remains to be known is how these agents would fit together sequentially into therapy if patients had multiple targetable mutations. Novel agents on the horizon include the anti-CD47 macrophage checkpoint molecule magrolimab, menin inhibitors, and immunotherapies such as antibody-drug conjugates (vadastuximab talirine and IMGN632), bispecific antibodies (vibecotamab and flotetuzumab), and chimeric antigen receptor T cells.6
Maintenance Therapy
Relapse after remission occurs in more than 50% of adults with high-risk AML, so it is vital to develop therapies that can maintain remission and improve survival. The most effective modality to maintain remission is allogeneic HSCT; however, many older patients may not be candidates due to comorbidities, frailty, or caregiver issues.11 The 2020 American Society of Hematology guidelines for treating older adults with newly diagnosed AML recommend consideration of post-remission therapy over no additional therapy in patients who achieve remission after at least a single cycle of intensive anti-leukemic therapy and who are not candidates for allogeneic HSCT.3 In addition to improving OS, maintenance therapy should aim to eradicate MRD, which has been shown to be the source of most relapses after remission.11 MRD is quantified by several different methods; however, the lack of standardization in assays and reporting make the results difficult to compare between trials. MRD can be used as a prognostic tool, a monitoring tool to predict relapse, and a surrogate end point for OS in future clinical trials. Per the European LeukemiaNet MRD Working Party consensus document, MRD should be assessed at diagnosis to enable comparison of biomarkers after patients have received treatment. Depending on the baseline genomic profile, MRD assessments are done at specific timepoints throughout therapy, and potential follow-up is conducted to determine response to therapy and predict impending relapse.35
Approaches to maintenance therapy for AML after remission have included cytotoxic chemotherapy, hypomethylating agents, immunotherapy, and targeted therapy. Evidence in favor of maintenance therapies is limited, as, regardless of agent or schedule, multiple trials have shown no OS benefit or decrease in relapse rate, including EORTC-HOVON, LAME Group, HOVON97, ECOG-ACRIN E2906, UK NCRI AML16, CALGB 9720, and UK MRC AML11.11
Hypomethylating Agents
IV azacitidine and decitabine have been studied as maintenance therapies after achievement of CR in patients who are not eligible for HSCT. The HOVAN97 phase 3 trial randomized patients to azacitidine 50 mg/m2 on days 1 to 5 every 4 weeks for 12 cycles versus placebo. Patients were 60 years or older with AML or MDS in CR/CRi after at least 2 cycles of intensive chemotherapy. Median disease-free survival (DFS) was higher in the study arm at 12 months (64% vs 42%; P = .04) but statistically significant improvement in OS was not seen.36 Decitabine was studied in the randomized, phase 2 trial ECOG-ACRIN E2906. Patients were 60 years or older with AML in CR/CRi after induction and consolidation therapy. Patients in the study arm received decitabine days 1 to 3 every 28 days for 1 year versus a control arm solely on observation. Median DFS and OS were higher in the decitabine arm, but the study was closed below targeted accrual and neither end point reached statistical significance.37
Oral alternatives or oral equivalents of hypomethylating agents are of interest to decrease time and cost spent on healthcare visits to administer IV chemotherapy. QUAZAR-AML-001 was a phase 3, randomized, double-blind, placebo-controlled trial of the oral formulation of azacitidine. Oral azacitidine or placebo was given once daily for 14 days each 28-day cycle to patients with AML who were 55 years or older and in CR after intensive chemotherapy. The primary end point was median OS, and secondary end points included DFS and health-related QOL. Median OS was 24.7 months with oral azacitidine versus 14.8 months with placebo (P <.001). Median relapse-free survival was higher in the study arm (10.15 vs 4.83 months; P <.001), and azacitidine was noninferior to placebo for health-related QOL. However, a higher rate of vomiting occurred in patients on oral azacitidine (60% vs 10%) especially during cycles 1 and 2. Patients should take a serotonin (5-HT3) receptor antagonist such as ondansetron approximately 30 minutes before each dose of oral azacitidine to mitigate risk of nausea and vomiting. Clinicians may consider observation versus initiation of maintenance therapy in patients with history of intractable nausea and vomiting.38 Based on the results of this trial, oral azacitidine was FDA approved for maintenance therapy after achievement of first remission following intensive induction chemotherapy in patients who are not able to complete intensive curative therapy.39
Targeted Agents
Targeted agents for AML are promising options for maintenance therapy, as these medications cause minimal myelosuppression when compared with other anti-leukemia therapies. The efficacy of targeted agents in maintenance is largely unclear, as many studies include the drug in induction, consolidation, and maintenance therapy. If patients are unfit for induction and consolidation therapy, the role of targeted therapy as maintenance is less defined.11
FLT3 inhibitors have been studied most frequently for maintenance indications. Sorafenib was added to intensive standard-of-care chemotherapy in older patients with FLT3-positive AML; patients continued on sorafenib for up to 1 year following induction. The study arm failed to reach its primary end point, as no difference was shown between groups for median event-free survival. Study authors indicated that excess toxicity in the sorafenib arm led to high rates of infectious mortality and lower protocol adherence for maintenance therapy.40 Midostaurin maintenance was analyzed in the RATIFY trial after patients in the study arm received midostaurin for 2-week courses with each cycle of induction and consolidation, followed by continuous therapy for 12 months. The trial showed significant improvement in OS and event-free survival, leading to FDA approval of midostaurin in combination with induction and consolidation chemotherapy for FLT3-mutated AML.41 However, a post hoc analysis of the trial failed to show definitive benefit of maintenance midostaurin due to no difference in DFS or OS from time of starting maintenance, thus midostaurin is not FDA approved for maintenance.42
Managed Care Considerations
Decision making regarding treatment modality and choice of therapy is complex in older patients with AML. Patient-specific challenges due to age-related medical, social, and functional factors may reduce patients’ ability to tolerate conventional cytotoxic induction chemotherapy. Disease in older adults differs from younger patients with AML, typically bearing higher rates of cytogenic aberrations and unfavorable genetic mutations that may cause resistance to traditional treatments.3 Finally, physicians and patients may be hesitant to initiate treatment for AML due to perceived risk versus benefit. Many older adults value QOL over length of life, so treatment decisions should be personalized for each patient, weighing consensus guidelines, benefits and toxicities of treatments, patient-specific factors, and, most importantly, patient goals of care.3,43
Treatment in Older Patients With Acute Myeloid Leukemia
Patient-specific medical factors include age-related decline in organ function, potential for serious preexisting comorbidities, and performance status that might not correspond with physiologic age.3 In a population-based study performed in Sweden, higher mortality rates were observed in patients with history of cerebrovascular, rheumatologic, renal, liver, and psychiatric disease. Renal dysfunction and disorders were associated with the highest increase in all-cause and AML-specific death. In addition, dementia was associated with AML-specific mortality.44
Assessing fitness for treatment is an extremely important aspect of clinical decision making in the elderly population. Recent trials in elderly or unfit patients have used consensus criteria established by Ferrari, et al, deeming a patient unfit for intensive chemotherapy if they fulfilled 1 or more of the following criteria: severe cardiac comorbidity, severe pulmonary comorbidity, severe renal comorbidity, severe hepatic comorbidity, active infection resistant to anti-infective therapy, cognitive impairment, low performance status, or any other comorbidity that the physician judges to be incompatible with chemotherapy.45 A comprehensive geriatric assessment evaluates multiple areas to determine fitness or frailty, including physical function, comorbid disease, cognitive function, psychological state, social support, polypharmacy, and nutritional status.46 Geriatric assessments are recommended by NCCN and American Society of Clinical Oncology (ASCO) guidelines, and these types of assessments can provide guidance for nonintensive therapy decisions.47 In an observational study of patients receiving treatment or best supportive care for MDS or AML, 3 variables were independently associated with worse survival: requiring assistance with activities of daily living, high fatigue score, and impaired performance status.48 Regardless of the specific tool used, evaluating fitness for treatment is necessary to tailor the therapy plan.
AML in older patients can be difficult to treat due to inherent differences in the disease biology compared with younger patients. Older patients have increased expression of multidrug resistance (MDR1 genes), higher prevalence of secondary AML, higher prevalence of unfavorable molecular and cytogenetic abnormalities, and decreased sensitivity to anthracyclines.7,49-51 Older patients show a decreased response to traditional chemotherapy due to these factors, leading to lower rates of remission, higher rates of complications, and inferior DFS and OS.3
Economic Burden
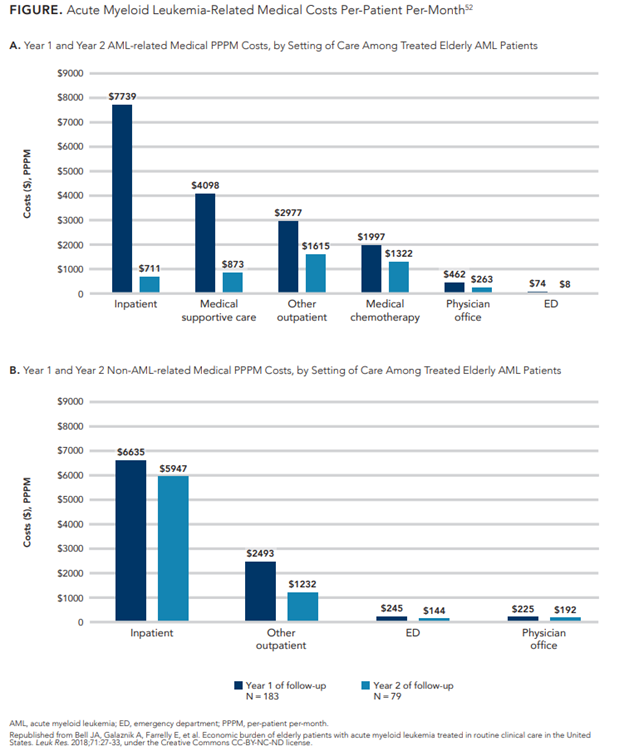
The treatment and supportive care of AML is associated with high healthcare costs and extensive healthcare utilization. Patients require frequent outpatient visits, numerous hospitalizations, and large amounts of blood products, including red blood cell and platelet transfusions, in addition to other supportive care services.52 Over a quarter of patients with AML require intensive care unit-level care, which can add significant cost to an already large economic burden.53 A retrospective claims database study examining healthcare utilization and costs associated with AML in patients older than 60 years demonstrated substantial costs in the first year of treatment. Total mean per-patient per-month (PPPM) costs were $27,756 (standard deviation: $22,121), with the majority of costs associated with medical treatments. Inpatient hospitalization was the largest cost driver, accounting for 45% of total medical costs in the first year at $7739 PPPM (Figure52). Ninety-two percent of patients in this analysis were hospitalized at least 1 time during the study period. Pharmacy costs, including chemotherapy, supportive care, and non-AML−related costs, totaled only $780 PPPM, likely due to the lack of chemotherapeutic and targeted options during the study time period (2007-2015).52 Pharmacy-related costs, specifically for outpatient prescription therapy to both treat disease and prevent infectious complications, have increased dramatically in the past 5 years due to recent approvals of oral agents. Patients with private insurance may face substantial costs for co-payments, and those on Medicare may experience challenges in access.54
Another retrospective cohort study evaluated claims data to determine healthcare resource utilization and direct healthcare costs, as well as the relationship with clinical outcomes for patients with AML. The study defined episodes by treatments, including high-intensity chemotherapy regimens, low-intensity chemotherapy (LIC), and HSCT. LIC was defined as LDAC, anthracycline, azacitidine, decitabine, clofarabine, or gemtuzumab ozogamicin administered in the outpatient setting. Of note, none of these data included the current NCCN-preferred regimens for patients 60 years or older determined unfit for intensive chemotherapy due to the date of analysis (2008-2016). Mean total costs for LIC were $53,081, and 35.8% of patients had at least 1 inpatient hospitalization during the study period, costing a mean of $49,580.55 As both studies demonstrated the primary driver of medical costs to be inpatient hospitalization, optimization of new outpatient-based regimens to prevent unplanned inpatient hospitalizations may be key to minimizing healthcare costs.
Oncology Clinical Pathways
Clinical pathways are structured, multidisciplinary care plans that detail steps in the care of patients with a specific clinical problem. Currently, more than 170 million patients, many of whom may be receiving treatment for cancer, are potentially being treated under insurance plan-sponsored pathways.56 Oncology clinical pathways (OCPs) are detailed protocols for delivering cancer care to ensure consistency of evidence-based care, manage medication use, decrease payer appeals and drug prices, and promote accrual to clinical trials. OCP protocols may include drug regimens for specific patient populations depending on type, stage, and molecular subtype of disease. To improve the development of OCPs by provider- and payer-marketed pathway vendors as well as decision support tool vendors, the ASCO Policy Statement on OCP recommends a collaborative, national approach to remove the unsustainable administrative burdens associated with multiple, disparate OCPs. In addition, the policy statement recommends that all OCPs57:
- Should be developed through a process that is consistent and transparent
- Should address the full spectrum of cancer care, from diagnosis to survivorship and end-of-life care
- Should promote the best possible evidence-based care that is updated continuously
- Should recognize patient variability and autonomy
- Should be implemented in ways that promote administrative efficiencies for both oncology providers and payers
- Should promote education, research, and clinical trial access
For OCPs to realize reduction in costs while improving the overall quality of care, exacting questions should be answered to evaluate clinical pathways. ASCO has developed a guide for evaluating OCPs to determine if current or potential future pathways are of high quality. Three areas of evaluation are development, implementation and use, and analytics. Development criteria include the following: OCP created by oncology experts reflecting stakeholder input; clear process and transparency of OCP creation; OCP is evidence based, patient focused, clinically driven, timely, and comprehensive in scope; and OCP promotes participation in clinical trials. OCP implementation and use criteria revolve around the information provided to as well as support and communication offered to participant practices; OCPs should have clear, achievable expected outcomes, integrated cost-effective technology and decision support, and efficient processes for communication adjudications. The final criteria are analytics; establishing OCPs should provide efficient and public reporting of performance metrics, should have outcomes-driven results, and should promote research and continuous quality improvement.58
As AML treatment options expand and more targeted agents are FDA approved, an OCP to manage older patients with AML will be useful to providing high-efficacy, lower-toxicity treatments.59 Several studies in solid tumor populations indicate that use of pathways can reduce costs while either maintaining or improving the quality of care, demonstrating up to 30% to 35% cost savings with equivalent outcomes.58 A large, independent, oncologist-led practice collaborated with Aetna to conduct a payer-sponsored program using evidence-based pathways in 746 Medicare Advantage patients diagnosed with cancer. Over 3 years, cumulative cost savings were over $3 million; drug cost savings per-patient per-treatment per-month were $1874. Interestingly, drug cost savings were largely driven by solid tumor indications. Few savings were realized in patients with hematologic malignancies.60 Developing pathways for AML will likely involve differentiating AML into subsets based on mutational profile and patient-specific factors, then selecting the most effective therapy for that indication based on high-quality, updated evidence. If options are equivalent in efficacy, choosing the option with less toxicity should be prioritized. If risk of toxicity is equivalent among options, the treatment with the lowest cost and/or more economic value should be chosen.61
The Pharmacist’s Role
Pharmacists are uniquely poised to address the unique needs of older patients with AML. Preferred treatment regimens may be complex for many older patients to navigate. Many regimens involve both IV and oral agents that have different dosing schedules and may be frequently modified based on patients’ laboratory values or AEs. IV products are administered in a healthcare setting, whereas oral agents typically must be procured via specialty pharmacies.62 Prior authorization, high co-pays, and shipment of medications add an extra layer of complexity for older patients in receiving care in a timely and appropriate manner.63 Pharmacists can help by alleviating barriers to access of medication, optimizing use of medications based on patient-specific factors and goals of care, improving adherence, and helping manage AEs.64 Venetoclax-based regimens are especially complex due to frequent need to modify cycle length and start dates, as well as scheduling oral and IV chemotherapy in concert. Clinical pharmacists and specialty pharmacy support are essential to optimize care and reduce hospitalizations.65 In addition, many oral therapies for AML have extensive drug-drug interactions that may warrant dose adjustment as well as more frequent monitoring or closer follow-up. If patients are on other medications for comorbid conditions, pharmacists can address drug interactions and ensure patients are receiving the most optimal regimen for their entire medication and disease state history.62 Managed care pharmacists can improve patient care by optimizing best practices, including management of utilization, drug cost, medication therapy, formulary decisions, and medical-to-pharmacy benefit channels. Managed care pharmacists can also add value by helping to develop and analyze outcomes from OCPs.66
Conclusions
The treatment of AML in older adults is a rapidly changing landscape due to the introduction of novel agents and combinations that show a promise of long-term remission for a population that has historically not been offered, did not tolerate, or did not respond to conventional cytotoxic chemotherapy. Managed care organizations and pharmacists can help bridge the gap by recognizing the expanding indications of current drugs while staying up-to-date on new therapies, including targeted agents. As AML moves from a disease with a definitive induction, consolidation, and remission to one that may involve long-term treatments and maintenance therapies, managed care professionals must realize the nuances of these therapies to determine the value of these medications balanced with realistic outcomes, patients’ wishes, and goals of care.6,67
REFERENCES
- National Cancer Institute. Cancer stat facts: leukemia —acute myeloid leukemia (AML). Accessed June 16, 2022. https://seer.cancer.gov/statfacts/html/amyl.html
- Pulte D, Gondos A, Brenner H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica. 2008;93(4):594-600. doi:10.3324/haematol.12304
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Short NJ, Montalban-Bravo G, Hwang H, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv. 2020;4(22):5681-5689. doi:10.1182/bloodadvances.2020003120
- DiNardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematology Am Soc Hematol Educ Program. 2016;2016(1):348-355. doi:10.1182/asheducation-2016.1.348
- Roloff GW, Odenike O, Bajel A, Wei AH, Foley N, Uy GL. Contemporary approach to acute myeloid leukemia therapy in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1-16. doi:10.1200/EDBK_349605
- Almeida AM, Ramos F. Acute myeloid leukemia in older adults. Leuk Res Rep. 2016;6:1-7. doi:10.1016/j.lrr.2016.06.001
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. doi:10.1056/NEJMra1406184
- Loke J, Lowe DM, Miller LJ, et al; National Cancer Research Institute Acute Myeloid Leukaemia Working Party on Supportive Care/Transfusion. Supportive care in the management of patients with acute myeloid leukaemia: where are the research needs? Br J Haematol. 2020;190(3):311-313. doi:10.1111/bjh.16708
- Mort MK, Sen JM, Morris AL, et al. Evaluation of cardiomyopathy in acute myeloid leukemia patients treated with anthracyclines. J Oncol Pharm Pract. 2020;26(3):680-687. doi:10.1177/1078155219873014
- Reville PK, Kadia TM. Maintenance therapy in AML. Front Oncol. 2021;10:619085. doi:10.3389/fonc.2020.619085
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. doi:10.1200/JCO.2011.38.9429
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. doi:10.1182/blood-2015-01-621664
- Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116(13):2224-2228. doi:10.1182/blood-2010-02-270330
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Acute myeloid leukemia. (version 2.2022—June 14, 2022). Accessed June 16, 2022. www.nccn.org/professionals/physician_gls/pdf/aml.pdf
- DiNardo C, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359-367. doi:10.1016/j.clinthera.2017.01.003
- Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia. 2019;33:2795-2804. doi:10.1038/s41375-019-0612-8
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216-228. doi:10.1016/S1470-2045(18)30010-X
- Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208-217. doi:10.1002/ajh.26039
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Wang ES. Treating acute myeloid leukemia in older adults. Hematology Am Soc Hematol Educ Program. 2014;2014(1):14-20. doi:10.1182/asheducation-2014.1.14
- Inqovi. Prescribing information. Taiho Oncology, Inc; March 2022. Accessed June 16, 2022. https://taihocorp-media-release.s3.us-west-2.amazonaws.com/documents/INQOVI_Prescribing_Information.pdf
- Geissler K, Koristek Z, del Castillo TB, et al. Pharmacokinetic exposure equivalence and preliminary efficacy and safety from a randomized cross over phase 3 study of an oral hypomethylating agent dec-c compared to iv decitabine in AML patients. European Hematology Association. Abstract P573. Released May 12, 2022. Accessed June 16, 2022. https://library.ehaweb.org/eha/2022/eha2022-congress/357436
- DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984
- Roboz GJ, DiNardo CD, Stein EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463-471. doi:10.1182/blood.2019002140
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405
- Pollyea DA, Tallman MS, de Botton S, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575-2584. doi:10.1038/s41375-019-0472-2
- Fathi AT, DiNardo CD, Kline I, et al. Alterations in serum bilirubin during enasidenib treatment in patients with or without UGT1A1 mutations. J Clin Oncol. 2018;36(15 suppl). doi:10.1200/JCO.2018.36.15_suppl.e19003
- Idhifa. Prescribing information. Celgene Corporation; November 2020. Accessed June 16, 2022. https://packageinserts.bms.com/pi/pi_idhifa.pdf
- Tibsovo. Prescribing information. Servier Pharmaceuticals; May 2022. Accessed June 16, 2022. www.tibsovopro.com/pdf/prescribinginformation.pdf
- Zeidner JF. Differentiating the differentiation syndrome associated with IDH inhibitors in AML. Clin Cancer Res. 2020;26(16):4174-4176. doi:10.1158/1078-0432.CCR-20-1820
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Heuser M, Freeman SD, Ossenkoppele GJ, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138(26):2753-2767. doi:10.1182/blood.2021013626
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Foran JM, Sun Z, Claxton DF, et al. Maintenance decitabine (DAC) improves disease-free (DFS) and overall survival (OS) after intensive therapy for acute myeloid leukemia (AML) in older adults, particularly in FLT3-ITD-negative patients: ECOG-ACRIN (EA) E2906 randomized study. Blood. 2019;134(suppl 1):115. doi:10.1182/blood-2019-129876
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Onureg. Prescribing information. Celgene Corporation; May 2021. Accessed June 16, 2022. https://packageinserts.bms.com/pi/pi_onureg.pdf
- Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110-3118. doi:10.1200/JCO.2012.46.4990
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359
- Larson RA, Mandrekar SJ, Sanford BL, et al. An analysis of maintenance therapy and post-midostaurin outcomes in the international prospective randomized, placebo-controlled, double-blind trial (CALGB 10603/RATIFY [Alliance]) for newly diagnosed acute myeloid leukemia (AML) patients with FLT3 mutations. Blood. 2017;130(suppl 1):145. doi:10.1182/blood.V130.Suppl_1.145.145
- Loh KP, Abdallah M, Kumar AJ, Neuendorff NR, Dahiya S, Klepin HD. Health-related quality of life and treatment of older adults with acute myeloid leukemia: a young Internal Society of Geriatric Oncology review paper. Curr Hematol Malig Rep. 2019;14(6):523-535. doi:10.1007/s11899-019-00552-6
- Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma - a Swedish population-based study. BMC Cancer. 2015;15:850. doi:10.1186/s12885-015-1857-x
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Klepin HD, Estey E, Kadia T. More versus less therapy for older adults with acute myeloid leukemia: new perspectives on an old debate. Am Soc Clin Oncol Educ Book. 2019;39:421-432. doi:10.1200/EDBK_239097
- Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36:2326-2347. doi:10.1200/JCO.2018.78.8687
- Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208-216. doi:10.3324/haematol.2012.067892
- Rao AV, Valk PJ, Metzeler KH, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(33):5580-5586. doi:10.1200/JCO.2009.22.2547
- Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481-3485. doi:10.1182/blood-2005-09-3724
- Finn L, Dalovisio A, Foran J. Older patients with acute myeloid leukemia: treatment challenges and future directions. Ochsner J. 2017;17(4):398-404.
- Bell JA, Galaznik A, Farrelly E, et al. Economic burden of elderly patients with acute myeloid leukemia treated in routine clinical care in the United States. Leuk Res. 2018;71:27-33. doi:10.1016/j.leukres.2018.06.010
- Halpern, Culakova E, Walter RB, Lyman GH. Association of risk factors, mortality, and care costs of adults with acute myeloid leukemia with admission to the intensive care unit. JAMA Oncol. 2017;3(3):374-381. doi:10.1001/jamaoncol.2016.4858
- Forsythe A, Sandman K. What does the economic burden of acute myeloid leukemia treatment look like for the next decade? an analysis of key findings, challenges and recommendations. J Blood Med. 2021;12:245-255. doi:10.2147/JBM.S279736
- Pandya BJ, Chen CC, Medeiros BC, et al. Economic and clinical burden of acute myeloid leukemia episodes of care in the United States: a retrospective analysis of a commercial payer database. J Manag Care Spec Pharm. 2020;26(7):849-859. doi:10.18553/jmcp.2020.19220
- Clinical pathways. American Society of Clinical Oncology. Accessed July 12, 2022. www.asco.org/news-initiatives/current-initiatives/cancer-care-initiatives/clinical-pathways
- Zon RT, Frame JN, Neuss MN, et al. American Society of Clinical Oncology policy statement on clinical pathways in oncology. J Oncol Pract. 2016;12(3):261-266. doi:10.1200/JOP.2015.009134
- Zon RT, Edge SB, Page RD, et al. American Society of Clinical Oncology criteria for high-quality clinical pathways in oncology. J Oncol Pract. 2017;13(3):207-210. doi:10.1200/JOP.2016.019836
- Daly B, Zon RT, Page RD, et al. Oncology clinical pathways: charting the landscape of pathway providers. J Oncol Pract. 2018;14(3):e194-e200. doi:10.1200/JOP.17.00033
- Hoverman JR, Neubauer MA, Jameson M, et al. Three-year results of a Medicare Advantage cancer management program. J Oncol Pract. 2018;14(4):e229-e237. doi:10.1200/JOP.17.00091
- JCP Editors. How novel agents are likely to be incorporated into clinical pathways for acute myeloid leukemia. J Clin Pathways. May 2018. Accessed June 16, 2022. www.hmpgloballearningnetwork.com/site/jcp/article/how-novel-agents-are-likely-be-incorporated-clinical-pathways-acute-myeloid-leukemia
- Palmer S, Chen A, Dennison T, et al. Impact of oncology pharmacists on the knowledge, attitude, and practices of clinicians to enhance patient engagement of self-administered oral oncolytics. Pharmacy (Basel). 2021;9(3):130. doi:10.3390/pharmacy9030130
- Niccolai JL, Roman DL, Julius JM, Nadour RW. Potential obstacles in the acquisition of oral anticancer medications. J Oncol Pract. 2017;13(1):e29-e36. doi:10.1200/JOP.2016.012302
- Muluneh B, Schneider M, Faso A, et al. Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. J Oncol Pract. 2018;14(6):e324-e334. doi:10.1200/JOP.17.00039
- Buhlinger K, Valgus J, Alexander M, et al. Pharmacist-led program leads to safe and efficient outpatient initiation of AML venetoclax-based regimen. ASHP Best Practices Award. American Society of Health-System Pharmacists Midyear Clinical Meeting. Chapel Hill, NC. December 2021. Accessed July 11, 2022. www.ashp.org/-/media/assets/about-ashp/docs/best-practice-award/2021-bp-kaitlyn-buhlinger-nc.pdf
- Evolving strategies for cost-effective cancer management. Value Based Cancer Care. 2012;3(7).
- Tombleson R. Impact of emerging clinical trends on overall cost of care and the role of the managed care pharmacist. Am J Manag Care. 2021;27(5 suppl):S97-S103. doi:10.37765/ajmc.2021.88627
