- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
The Impact of PCSK9 Modulation on Cardiovascular Outcomes: Recent Advances and the Managed Care Implications
To claim CE credit for this activity, please visit http://www.pharmacytimes.org/pcsk9-ajmc
ABSTRACT
Cardiovascular disease (CVD) remains the leading cause of death globally. Hypercholesterolemia is a major modifiable risk factor for developing atherosclerotic CVD (ASCVD). Although statins are the foundational evidence-based treatment option, significant gaps in care exist as approximately 5% to 30% of patients do not tolerate statin therapy. Ezetimibe provides additional, but modest, reductions in low-density lipoprotein cholesterol (LDL-C) and ASCVD risk. The PCSK9 enzyme has emerged as a viable therapeutic target, resulting in the approval of 2 monoclonal antibodies, alirocumab and evolocumab, and a small interfering RNA molecule, inclisiran, that reduce LDL-C levels by approximately 60% and 50%, respectively. Alirocumab and evolocumab were approved in 2015 and have been shown to reduce ASCVD risk in secondary prevention patients; however, the cost of therapy has been a barrier to uptake despite significant price reductions. Inclisiran is unique in that it requires administration by a healthcare professional, thus creating challenges and unknowns when it comes to implementing this drug in clinical practice. Managed care professionals have considerable experience with developing approaches to providing access to novel injectable lipid-lowering therapies, such as alirocumab and evolocumab, and with the approval of inclisiran, they now have an expanding list of such therapies to incorporate into their care plans.
Am J Manag Care. 2022;28(suppl 8):S139-S147. https://doi.org/10.37765/ajmc.2022.89269
Introduction to Hypercholesterolemia: Epidemiology and the Burden of Disease
Cardiovascular disease (CVD) is the leading cause of death in the United States and worldwide.1,2 The burden of CVD mortality continues to increase alongside modifiable risk factors.1,3 Hypercholesterolemia is of particular concern given that elevated levels of low-density lipoprotein cholesterol (LDL-C) are a primary driver of atherosclerotic CVD (ASCVD).4 It is estimated that nearly 94 million adults in the United States have total cholesterol levels higher than 200 mg/dL, yet approximately half of all adults who could benefit from pharmacologic treatment are taking it.3
Evidence from Mendelian randomization studies, randomized controlled trials, and observational studies strongly supports the use of select LDL-C−lowering therapies to decrease the risk of ASCVD events.4 Since the approval of lovastatin in 1984, HMG-CoA reductase inhibitors (ie, statins) have been the primary therapy used to lower LDL-C−associated ASCVD risk. Even with optimal statin use, significant residual cardiovascular risk remains, and not all patients achieve desired LDL-C levels with statin therapy alone. Furthermore, approximately 5% to 30% of patients are unable to tolerate statin therapy.5 Nonstatin therapies can serve as suitable alternatives to either use in combination with maximally tolerated statin or instead of statins in cases of complete statin intolerance; however, not all the available nonstatins have been shown to reduce ASCVD events when used in combination with statin therapy.6
Unmet Needs in a Growing Population
Despite the significant body of evidence supporting the use of statins to reduce ASCVD events,7 they remain underutilized. A national outpatient registry of over 2.5 million patients with ASCVD reported that over 50% had no history of lipid-lowering therapy use. In those who were receiving statin monotherapy, approximately two-thirds did not achieve an LDL-C below 70 mg/dL.8 Another retrospective cohort study of over 600,000 commercially insured patients found less than a quarter of patients with ASCVD were receiving a high-intensity statin.9 This evidence strongly suggests that there are significant treatment gaps even among patients at highest ASCVD risk.
Another population in which large treatment gaps exist are patients with familial hypercholesterolemia (FH), a primary cause of premature ASCVD that affects as many as 1.5 million Americans.10 Patients with heterozygous FH typically have LDL-C levels of at least 190 mg/dL, though may exceed 400 mg/dL in rare cases, and may remain underdiagnosed and undertreated. An analysis of 1295 individuals with heterozygous FH from the CASCADE-FH Registry found patients with FH are diagnosed late in life, and just 25% of those being treated with lipid-lowering therapy achieved an LDL-C below 100 mg/dL.10 Thus, there is significant room for improvement in the management of patients with FH. Most patients with FH will require combination lipid-lowering therapy to achieve desired LDL-C levels.
Evidence-Based Treatment Guidelines and Advances in Pharmacotherapy
Hypercholesterolemia Treatment Guidelines from the American Heart Association and American College of Cardiology
Clinical practice guidelines are important tools to inform evidence-based practice and help address gaps in care. The most recent American Heart Association (AHA)/American College of Cardiology (ACC)/Multisociety Guideline on the Management of Blood Cholesterol was published in 2018.6 Statin therapy remains the first-line therapy to lower LDL-C and ASCVD risk. The decision to initiate statin therapy is largely driven by individual risk of ASCVD; the intensity of statin therapy increases with risk (Figure 1).6 For example, patients with established ASCVD should receive a high-intensity statin, while a moderate-intensity statin is recommended for primary prevention patients with an estimated 10-year ASCVD risk score of 7.5% to 19.9% and risk-enhancing factors. Low-intensity statins are generally not advised except in cases where moderate- or high-intensity statins are not tolerated.
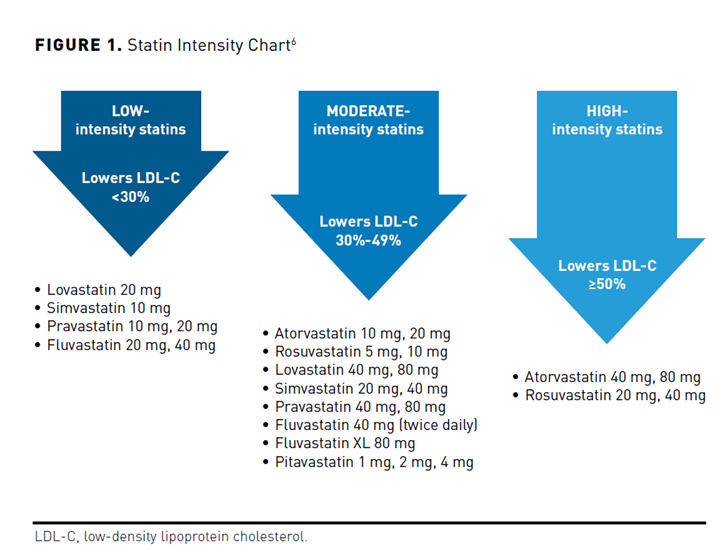
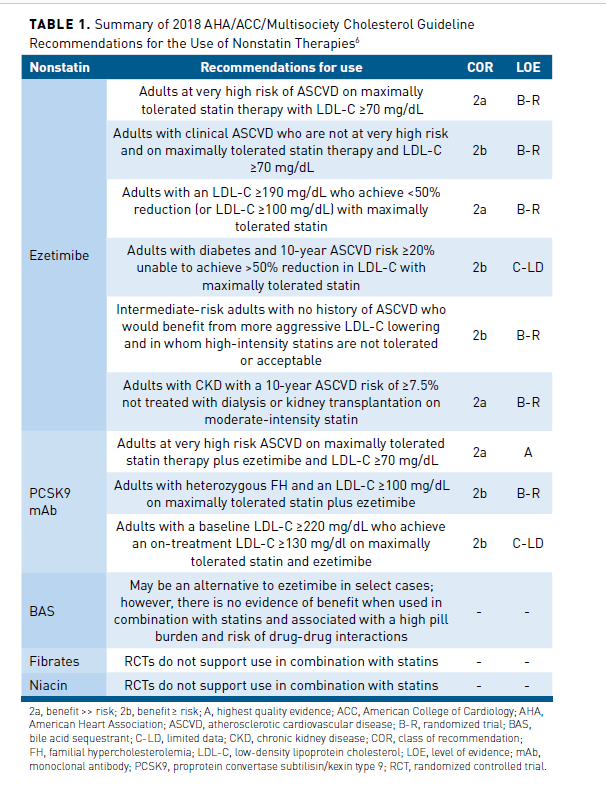
A major change in the 2018 guideline compared with the 2013 guideline11 was the inclusion of recommendations supporting the addition of ezetimibe and/or a proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody (mAb) to maximally tolerated statin therapy in select patients (Table 1).6 Bile acid sequestrants are also an option, but it is unknown if they reduce ASCVD risk when used in combination with statin therapy, are not well tolerated, and are associated with significant drug-drug interactions.6 Given that ezetimibe is an oral tablet administered once daily, generically available, has a strong safety record, and modestly reduces ASCVD event risk, ezetimibe is generally recommended before adding a PCSK9 mAb.6,12 Simulation studies have also shown that the majority (≈60%-80%) of secondary prevention patients will achieve an LDL-C less than 70 mg/dL with the combination of a high-intensity statin and ezetimibe.13,14 Nevertheless, the net clinical benefit with ezetimibe is modest, and not all patients will tolerate high-intensity statin therapy. A PCSK9 mAb may still be necessary to achieve treatment goals in select patients with established ASCVD, especially those with FH. While not approved at the time of the 2018 guideline, bempedoic acid can be used in combination with maximally tolerated statin in high-risk patients. Bempedoic acid reduces LDL-C by approximately 20% and inhibits ATP citrate lyase, a step in the cholesterol biosynthesis process that is upstream from HMG-CoA reductase.15 It is associated with fewer muscle-related adverse effects and is also available in a combination pill that includes ezetimibe.16 It is currently unknown if bempedoic acid reduces ASCVD risk.
Therapies Targeting the PCSK9 Enzyme
One of the major advances in lipid drug development was the discovery of PCSK9 in 2003.17 The PCSK9 enzyme is 1 of 9 proprotein convertases involved in various physiologic processes and is primarily expressed by hepatocytes where it binds to the epidermal growth factor-A domain of the LDL receptor (LDLR). This complex (PCSK9 = LDLR) enters the hepatocyte and is escorted to the lysosome by an unknown mechanism for degradation. This results in reduced availability of LDLR on the hepatocyte and prolonged circulation of LDL-C in the plasma, increasing the potential of it entering the arterial wall. This is further supported by genetic studies demonstrating that a gain-of-function in the PCSK9 gene produces hypercholesterolemia.18 Contrarily, if PCSK9 levels are low or its action on the LDLR inhibited, LDLR recycling increases and reduces the circulation of LDL-C in the plasma. As expected, a loss-of-function in PCSK9 produces hypolipidemia, supporting the potential for PCSK9 to be a successful drug target.19
The initial pharmacologic approach to inhibit the ability of PCSK9 to bind to extracellular LDLR was subcutaneously administered mAbs. This is because of the lack of a clear targetable groove in the complex (PCSK9 = LDLR) for small molecules (ie, oral medications) to bind to.17 Of course, other approaches are being developed to create an orally administered therapy, but these remain investigational.20,21 Two fully humanized mAbs targeting PCSK9, alirocumab and evolocumab, were approved by the FDA in 2015 (Table 2).22-25 A third agent, bococizumab, was also developed, but had greater immunogenicity and attenuation of LDL-C lowering over time and was associated with a greater risk of injection-site reactions than alirocumab and evolocumab.26
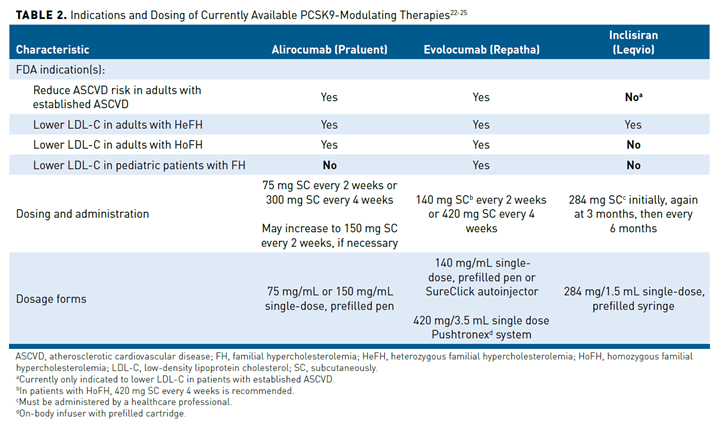
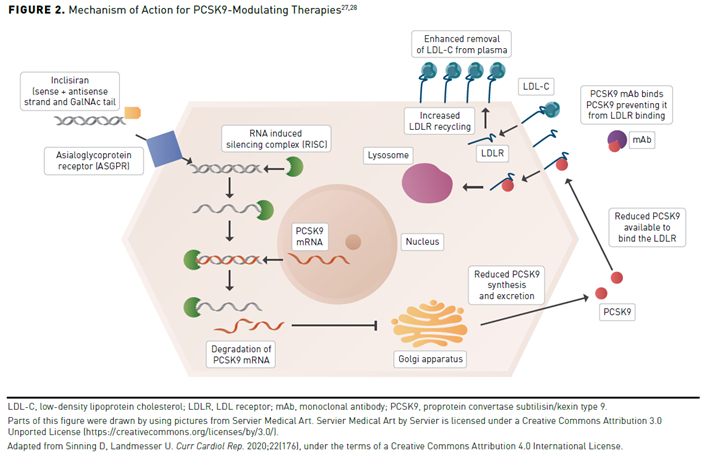
More recently, inclisiran, a small interfering RNA (siRNA) molecule against PCSK9, was approved by the FDA in 2021. Unlike the PCSK9 mAbs, inclisiran inhibits the production of intracellular PCSK9 through gene silencing (Figure 2).27,28 Inclisiran contains 2 nucleotide strands conjugated to a ligand that binds to a receptor found nearly exclusively on hepatocytes. Inclisiran is taken up by the hepatocyte via the asialoglycoprotein receptor and binds to the RNA-induced silencing complex, which cleaves the PCSK9 messenger RNA and prevents PCSK9 protein synthesis. The absence of PCSK9 enzymes prevents degradation of the LDLR, which is recycled to the hepatocyte service to reduce the circulation of LDL-C in the plasma. A major advantage of this approach is the long duration of the effect, which allows drug administration every 6 months instead of the biweekly or monthly administration of the PCSK9 mAbs (Table 2).22-25
Barriers to PCSK9 Treatment
A primary barrier to accessing PCSK9-targeted treatments is cost. When the PCSK9 mAbs initially came to market, the average wholesale price was approximately $15,000 per year.29 In contrast, all statins and ezetimibe are generically available, and therefore significantly more affordable for patients. They have proven to be cost-effective for the healthcare system.30,31 The initial cost of PCSK9 mAbs significantly limited their use, as prior authorizations (PAs) were required by third-party payers. In 2019, the annual cost of both alirocumab and evolocumab was reduced by 60% to approximately $5850 per year.32 Patient assistance programs are also available to help with access for patients meeting income requirements. Inclisiran is currently priced higher than the PCSK9 mAbs at $3250 per injection ($9750 for the first year, then $6500 annually thereafter); however, because inclisiran must be administered by a healthcare professional, the billing structure is different and presents its own challenges.27,33
An additional barrier is the subcutaneous administration route. It is well established that some patients are reluctant to self-administer or receive injectable medications.34 However, there are options for patients, as the PCSK9 mAbs can be administered biweekly or monthly, whereas inclisiran offers the advantage of every-6-month administration. Still, some patients will continue to reject injectable therapies, especially those with trypanophobia (fear of needles).34 Oral PCSK9-modulating therapies are in development, but they are unlikely to be available anytime in the near future.20,21
Recent Treatment Advances in Hypercholesterolemia
Several randomized controlled trials with nonstatin therapies were unsuccessful in showing a clinical benefit when added to background statin therapy, even among high-risk patients.35 This changed in 2015 when the IMPROVE-IT study demonstrated that ezetimibe provided a modest—but significant—reduction in major adverse cardiovascular events (MACE) over 7 years of follow-up when combined with simvastatin 40 mg daily.12 In 2017, the FOURIER trial demonstrated that evolocumab also reduced MACE by 15% in patients with stable ASCVD over 2.2 years of follow-up. One year later, the ODYSSEY trial also showed alirocumab reduced MACE by 15% in patients with a recent acute coronary syndrome (Table 322,36,37) over 2.8 years of follow-up.36-38 Although a 15% reduction in death from any cause was reported, a non-significant P value for death from coronary heart disease halted the hierarchical analysis plan, making this a nominal finding. Since publication of the original FOURIER and ODYSSEY trials, multiple analyses in various subgroups have also been published and demonstrated that the PCSK9 mAbs provide a significant clinical benefit.37,39-41 In fact, those with ASCVD plus other major risk factors and those with polyvascular ASCVD stand to benefit the most from PCSK9 mAb therapy.
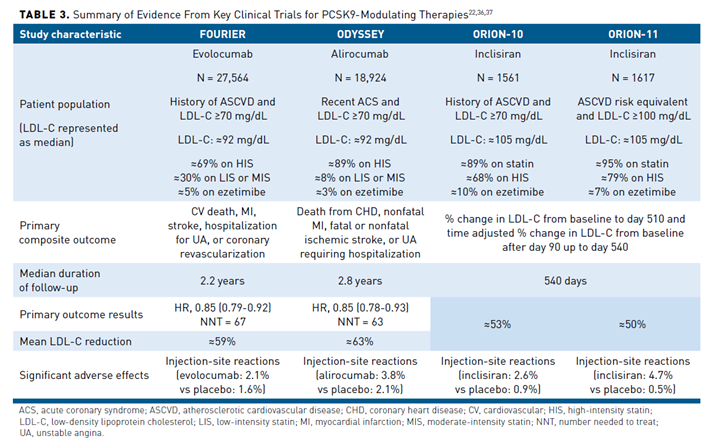
Safety is a major concern with any novel therapy, especially one capable of driving LDL-C to very low levels given the longstanding question of whether very low LDL-C may be harmful.42 Besides an increased risk of injection-site reactions, there were no major safety concerns observed in either the FOURIER or ODYSSEY trial, even in participants who achieved very low levels of LDL-C (<25 mg/dL).38,43 Furthermore, a prospective companion trial (EBBINGHAUS) to FOURIER showed no signs of cognitive impairment in patients receiving evolocumab.44 A Mendelian randomization study of approximately 740,000 participants further substantiated that PCSK9 inhibition is not associated with cognitive harm.45
The average follow-up in FOURIER and ODYSSEY was limited to 2 to 3 years, so the long-term safety is questionable. To address this, open-label extensions of both FOURIER and ODYSSEY have been conducted to provide additional data out to approximately 5 years of follow-up. Top-line results of 2 open-label extension studies from the FOURIER trial were announced in April 2022 and were seemingly positive, as 85% of patients achieved an LDL-C below 40 mg/dL and no safety concerns were identified; these data have yet to be published.46 The safety of high-intensity statins in people living with HIV has also been a longstanding concern given the increased ASCVD risk in this population as well as risk of drug-drug interactions.47 In the BEIJERINCK trial, evolocumab was shown to be safe and effective in people living with HIV taking maximally tolerated statin therapy without any increase in adverse effects.
Given the large body of safety and efficacy data, alirocumab and evolocumab are both indicated for use in patients with established ASCVD or FH who require additional LDL-C lowering despite maximally tolerated statin therapy.23,24 Only evolocumab, however, is approved for use in pediatric patients aged 10 years and older. Despite the favorable data and approved indications, the uptake of PCSK9 mAbs remains poor. A retrospective study using a national commercial claims database (2015-2019) found that less than 1% of patients with established ASCVD and an LDL-C greater than or equal to 70 mg/dL who were adherent to a high-intensity statin received a PCSK9 mAb.48 Similar findings were also observed in an analysis of real-world electronic health record data from the National Patient-Centered Clinical Research Network in more than 3.6 million patients.49 The use of PCSK9 mAbs in Medicare Part D beneficiaries is also low but increased steadily between 2015 and 2018.50 The average spending per beneficiary on alirocumab and evolocumab was $8746 and $7267, respectively; while this is significant, it was prior to the approximate 60% price reduction in 2019.50
A major advancement in the PCSK9 story is inclisiran, which was approved in 2021 and has been extensively evaluated in various populations as part of the ORION clinical trial program. The ORION-10 (patients with ASCVD) and ORION-11 (patients with ASCVD or ASCVD risk equivalent) trials were jointly published and demonstrated inclisiran lowers LDL-C by approximately 50%, with injection-site reactions being the only notable adverse effect.22 These data also demonstrated that inclisiran provides a consistent reduction in LDL-C levels with every 6-month administration, after an initial dose and followed by another at day 90. Currently, inclisiran is approved for use in combination with maximally tolerated statin in patients with heterozygous FH or ASCVD who require further lowering of LDL-C levels.25 What remains unknown is the impact of inclisiran on cardiovascular morbidity and mortality. The ongoing ORION-4 trial will randomize approximately 15,000 participants to inclisiran or placebo in those aged 55 years and older with prior myocardial infarction, ischemic stroke, or peripheral artery disease to determine if inclisiran reduces MACE. The ORION-4 trial is expected to have primary results in 2026.51
Managed Care Protocols to Reduce the High Burden of Hypercholesterolemia
The rising healthcare costs associated with managing patients with CVD are a major concern. Currently, the total cost of CVD exceeds $550 billion annually and is expected to exceed $1 trillion annually in 2035.52 Unsurprisingly, statins remain the most prescribed medication in the United States and are cost-effective. Recently approved pharmacotherapy options to treat hypercholesterolemia may provide additional clinical benefit to patients but come at a significantly higher cost than generically available statins. Strategies are needed to manage the increased demand for novel therapies to treat hypercholesterolemia and reduce the burden of CVD in the United States.
Team-Based Care
One key strategy to improve the management of hypercholesterolemia is to expand team-based care, which is generally defined as 2 or more healthcare professionals from different disciplines working collaboratively with patients to achieve shared goals.53 Team-based care is highly recommended by current clinical practice guidelines and the National Academy of Medicine.6,53 Importantly, there is significant evidence demonstrating that team-based care, particularly pharmacist interventions, is effective in improving hypercholesterolemia-related outcomes. Pharmacists can have wide-ranging responsibilities in both inpatient and outpatient settings that may involve medication optimization, reducing adverse drug events, and improving transitions of care through medication reconciliation and patient education.54 A recent meta-analysis demonstrated involving pharmacists in lipid management reduces LDL-C levels significantly more than usual care.55 Furthermore, team-based care can increase utilization of appropriate intensity statins in high-risk patients, which may reduce the need for nonstatin therapies, thereby reducing healthcare costs.56
Reinstating Lipid Monitoring as a Performance Measure
Since the 2013 ACC/AHA Cholesterol Guidelines recommended fixed-dose statins based on individual risk and found little evidence to support the use of available nonstatin therapies at the time, there has been significant confusion regarding the role of lipid monitoring.11 Although the 2013 guideline still recommended lipid monitoring (class 1a recommendation), it stated that, “LDL-C levels and percent reduction are to be used only to assess response to therapy and adherence. They are not to be used as performance standards.”11 This led to the assumption that lipid monitoring was unnecessary and resulted in the removal of LDL-C levels as a performance measure.57 Consequently, the current performance measure only addresses whether or not a patient received a statin and whether at least 80% or greater adherence has been achieved, not the level of LDL-C reduction achieved.58
The 2018 AHA/ACC/Multisociety Cholesterol Guidelines updated recommendations regarding the use of select nonstatin therapies (eg, ezetimibe, PCSK9 mAbs) given new evidence demonstrating their efficacy when used in combination with background statin therapy.6 These guidelines also introduced LDL-C thresholds for considering when to add nonstatins in high-risk patients but did not recommend specific goals of therapy, per se. Lipid monitoring again received a class 1a recommendation, but the assumption that lipid monitoring was unnecessary remained common among clinicians.
Since 2018, however, several studies have highlighted the importance of lipid monitoring. In a cohort of approximately 870,000 patients with ASCVD from the Veterans Affairs Healthcare System adherence (measured by prescription days covered) to statin therapy was significantly greater in patients who received lipid monitoring 4 to 12 weeks after initiation.59 This observation was also confirmed in other cross-sectional studies performed at Kaiser Permanente60 and with data from the Patient and Provider Assessment of Lipid Management Registry.61 This is critically important given that poor adherence to statin therapy is associated with an increased risk of ASCVD events and mortality.62 Another study with a more diverse patient population at an academic medical center found that 88% of patients receiving lipid-lowering therapy had received lipid monitoring within the past 12 months; however, lipid monitoring was significantly less likely to occur in those identifying as Black or African American, adults older than 75 years, and those insured by Medicaid.63 Lipid monitoring remains an important tool to ensure adherence and can help identify patients who may benefit from additional LDL-C lowering.
Value-Based Purchasing
In 2015, the Institute for Clinical and Economic Review (ICER) concluded that PCSK9 mAbs provide a significant clinical benefit, but the cost-effectiveness ratios exceeded $100,000 per quality-adjusted life year (QALY), a commonly accepted threshold for determining cost-effectiveness.64 A price reduction of at least 60% was recommended to achieve $100,000/QALY, which was implemented by both manufacturers of the PCSK9 mAbs in 2019; however, ICER stated an 85% reduction would be needed to achieve the value-price benchmark. Numerous cost-effectiveness studies have been published using various methodologies but generally agree that the PCSK9 mAbs are most cost-effective in the highest risk patients. A reasonable approach is to use the PCSK9 mAbs in patients who continue to have an LDL-C greater than or equal to 70 mg/dL despite maximally tolerated statin, plus ezetimibe as recommended in the 2018 AHA/ACC/Multisociety Cholesterol Guidelines.6
Although the inclisiran clinical outcomes trial (ORION-4) is still ongoing at this time, ICER conducted a comparative effectiveness analysis of inclisiran and bempedoic acid.65 ICER concluded that inclisiran substantially reduces LDL-C levels, and there were no major safety concerns. At the time, though the price of inclisiran had not yet been announced, ICER determined the health-benefit price benchmark range to be $3600 to $6000 per year. Another study used a lifetime Markov model and determined inclisiran would likely be cost-effective at the publicly available price ($3250 per dose), with a cost-effectiveness ratio of $51,686/QALY.66 A more conclusive cost-effectiveness analysis will have to await outcomes from the ORION-4 trial.
Structuring Reimbursement and Prior Authorization
Management of hypercholesterolemia is becoming increasingly complex with novel therapies rapidly coming to market. Decisions surrounding reimbursement and coverage of these novel therapies must consider the magnitude of the problem, potential safety concerns, and available clinical data supporting their efficacy, all of which are rapidly evolving.
Statin therapy remains the first-line treatment option for hypercholesterolemia, and most patients will tolerate the statin intensity indicated. However, it is commonly acknowledged that anywhere from 5% to 30% of patients will develop either partial or complete statin intolerance and require nonstatin therapies to achieve desired levels of LDL-C.5 Step therapy remains a reasonable approach. Adding generic ezetimibe to maximally tolerated statin therapy will achieve treatment goals in most patients and is considered a cost-effective strategy. Ezetimibe should generally be considered before adding bempedoic acid or PCSK9-modulating therapies. The PCSK9 mAbs should be reserved for patients with established ASCVD or FH who are unable to achieve treatment goals with maximally tolerated statin plus ezetimibe. Until evidence is available demonstrating that inclisiran and bempedoic acid reduce ASCVD events, these therapies should generally be reserved for patients unable to tolerate a PCSK9 mAb or in rare cases of inadequate LDL-C lowering. Another consideration for use may be those who are unable to perform self-injections or who would not be able to adhere to a biweekly or monthly regimen. Nonadherence to any PCSK9-modulating therapy must be avoided at all costs given the expense of these therapies. The VICTORION REAL study is currently underway to determine if every-6-month dosing with inclisiran improves adherence compared with standard of care.67
PA remains a frequently used cost-containment tool, but the health and economic impact of PAs is not well understood.68 After the initial approval of PCSK9 mAbs, approximately 60% of prescription claims were not approved.69 Delay and abandonment in approval of PCSK9 mAbs was also found to be associated with an increased risk of ASCVD events.69 Furthermore, PCSK9 mAb rejection rates are reportedly higher in women, racial minorities, and lower income groups. It should be noted that some clinicians were also not adhering to PA requirements, such as having a recent (<30 days) lipid panel on file, appropriately documenting statin intolerance, and trialing ezetimibe first.70,71 Involvement of pharmacists, especially specialty pharmacy, has improved patient access and helped patients navigate affordability.72,73 While PCSK9-modulating therapies are likely to continue requiring a PA until the costs of these therapies decline further, it is imperative that managed care professionals work to develop ways to increase patient access.
Inclisiran presents its own challenges when it comes to PA and patient accessibility, as it must be administered by a healthcare professional in the clinic, which is new for many.25 Inclisiran will likely be purchased through the “buy-and-bill” model.74 This means the medical practice or healthcare system purchases the drug to administer in the office instead of sending the prescription to an outpatient pharmacy for the patient to pick up. Specialty pharmacies may also serve as important partners in this process, as they can assist with coordinating shipment of the drug to the clinic so it is available for administration. Until such processes are developed and implemented at institutions, patient access to inclisiran could be limited.
Conclusions
Hypercholesterolemia remains a major modifiable risk factor for ASCVD, the leading cause of death globally. Reducing LDL-C is one of, if not the most important strategy to reduce ASCVD risk. Though statin therapies are among the most well studied drugs and are strongly supported by randomized controlled trials and clinical practice guidelines, not all patients will tolerate statin therapy or achieve sufficient LDL-C lowering with statin therapy alone. Ezetimibe remains the preferred initial nonstatin to add to maximally tolerated statin, but at least one-third of patients will require additional LDL-C lowering. The PCSK9 mAbs significantly lower LDL-C by approximately 60% and have been shown to be well tolerated and effective in reducing ASCVD events in high-risk groups. Inclisiran, a novel siRNA therapy targeting PCSK9, offers convenience as it requires administration every 6 months (by a healthcare professional) and reduces LDL-C by approximately 50%. The cost-effectiveness of PCSK9 mAbs and inclisiran remains in flux as pricing structures and the available clinical data continue to evolve. Until these factors become clearer, effective strategies are needed to ensure those most likely to benefit from a PCSK9-modulating therapy can receive it in accordance with current clinical practice guidelines.
Author affiliation: Dave L. Dixon, PharmD, FACC, FAHA, FCCP, FNLA, BCACP, CDCES, CLS, is Nancy L. and Ronald H. McFarlane Professor of Pharmacy and Chair of the Department of Pharmacotherapy & Outcomes Science at the Virginia Commonwealth University School of Pharmacy, Richmond, VA.
Funding source: This activity is supported by an educational grant from Novartis.
Author disclosure: Dr Dixon has no relevant financial relationships with commercial interests to disclose.
Author information: Substantial contributions to concept and design, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Address correspondence to: dldixon@vcu.edu
REFERENCES
- Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982-3021. doi:10.1016/j.jacc.2020.11.010
- Leading causes of death. Centers for Disease Control and Prevention. Published January 13, 2022. Accessed August 4, 2022. www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8). doi:10.1161/CIR.0000000000001052
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. evidence from genetic, epidemiologic, and clinical studies. a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. doi:10.1093/eurheartj/ehx144
- Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol. 2022;16(4):361-375. doi:10.1016/j.jacl.2022.05.068
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J Am Coll Cardiol. 2019;73(24):e285-e350. doi:10.1016/j.jacc.2018.11.003
- Wang N, Fulcher J, Abeysuriya N, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327,037 participants. Lancet Diabetes Endocrinol. 2020;8(1):36-49. doi:10.1016/S2213-8587(19)30388-2
- Allen JM, Arnold SV, Lohr NL, et al. Abstract 12904: assessing low-density lipoprotein cholesterol risk in secondary prevention patients within the PINNACLE National Outpatient Registry. 2019;140(Suppl_1):A12904. www.ahajournals.org/doi/10.1161/circ.140.suppl_1.12904
- Nelson AJ, Haynes K, Shambhu S, et al. High-intensity statin use among patients with atherosclerosis in the U.S. J Am Coll Cardiol. 2022;79(18):1802-1813. doi:10.1016/j.jacc.2022.02.048
- deGoma EM, Ahmad ZS, O’Brien EC, et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH Registry. Circ Cardiovasc Genet. 2016;9(3):240-249. doi:10.1161/CIRCGENETICS.116.001381
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 PART B):2889-2934. doi:10.1016/j.jacc.2013.11.002
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
- Virani SS, Akeroyd JM, Nambi V, et al. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the FOURIER trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk): insights from the Department of Veterans Affairs. Circulation. 2017;135(25):2572-2574. doi:10.1161/CIRCULATIONAHA.117.028503
- Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(9):959. doi:10.1001/jamacardio.2017.2289
- Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022-1032. doi:10.1056/NEJMoa1803917
- Agboola F, Lin GA, Kazi DS, McKenna A, Pearson SD. The effectiveness and value of bempedoic acid and inclisiran for heterozygous familial hypercholesterolemia and secondary prevention of ASCVD: A summary from the Institute for Clinical and Economic Review’s Midwest Comparative Effectiveness Public Advisory Council. J Manag Care Spec Pharm. 2021;27(7):961-966. doi:10.18553/jmcp.2021.27.7.961
- Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9: A key modulator of cardiovascular health. Circ Res. 2014;114(6):1022-1036. doi:10.1161/CIRCRESAHA.114.301621
- Cameron J, Holla ØL, Ranheim T, Kulseth MA, Berge KE, Leren TP. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum Mol Genet. 2006;15(9):1551-1558. doi:10.1093/hmg/ddl077
- Cohen JC, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264-1272. doi:10.1056/NEJMoa054013
- Mullard A. Merck readies oral, macrocyclic PCSK9 inhibitor for phase II test. Nat Rev Drug Discov. 2022;21(9):1. doi:10.1038/d41573-021-00195-4
- Salaheldin TA, Godugu K, Bharali DJ, Fujioka K, Elshourbagy N, Mousa SA. Novel oral nano-hepatic targeted anti-PCSK9 in hypercholesterolemia. Nanomedicine Nanotechnol Biol Med. 2022;40:102480. doi:10.1016/j.nano.2021.102480
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507-1519. doi:10.1056/NEJMoa1912387
- Praluent. Prescribing information. Regeneron; 2021. Accessed July 6, 2022. www.regeneron.com/downloads/praluent_pi.pdf
- Repatha. Prescribing information. Amgen; 2021. Accessed July 6, 2022. www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/repatha/repatha_pi_hcp_english.pdf
- Leqvio. Prescribing information. Novartis; 2021. Accessed July 6, 2022. www.novartis.us/sites/www.novartis.us/files/leqvio.pdf
- Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376(16):1527-1539. doi:10.1056/NEJMoa1701488
- Smith KW, White CM. Inclisiran: a novel small interfering RNA drug for low-density lipoprotein reduction. J Clin Pharmacol. 2022;62(9):1079-1085. doi:10.1002/jcph.2045
- Sinning D, Landmesser U. Low-density lipoprotein-cholesterol lowering strategies for prevention of atherosclerotic cardiovascular disease: focus on siRNA treatment targeting PCSK9 (inclisiran). Curr Cardiol Rep. 2020;22(176). doi:10.1007/s11886-020-01427-6
- Kazi DS, Lu CY, Lin GA, et al. Nationwide coverage and cost-sharing for PCSK9 inhibitors among Medicare Part D plans. JAMA Cardiol. 2017;2(10):1164. doi:10.1001/jamacardio.2017.3051
- Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142. doi:10.1001/jama.2015.6822
- Sasidharan A, Bagepally BS, Kumar SS, Jagadeesh KV, Natarajan M. Cost-effectiveness of ezetimibe plus statin lipid-lowering therapy: a systematic review and meta-analysis of cost-utility studies. Khan MS, ed. PLoS ONE. 2022;17(6):e0264563. doi:10.1371/journal.pone.0264563
- Robinson JG, Jayanna MB, Brown AS, et al. Enhancing the value of PCSK9 monoclonal antibodies by identifying patients most likely to benefit. J Clin Lipidol. 2019;13(4):525-537. doi:10.1016/j.jacl.2019.05.005
- Dixon DL, Saseen JJ. Pharmacist-administered long-acting injectable PCSK9 service: a solution to improve patient access and adherence. J Am Pharm Assoc. 2021;61(3):e83-e85. doi:10.1016/j.japh.2020.12.009
- McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J Adv Nurs. 2019;75(1):30-42. doi:10.1111/jan.13818
- Sisson EM, Pamulapati L, Bucheit JD, Kelly MS, Dixon DL. Evolving role of non-statin therapy for the management of dyslipidemia and cardiovascular risk reduction: past, present, and future. Pharmacotherapy. 2018;38(2):164-171. doi:10.1002/phar.2074
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
- Jukema JW, Szarek M, Zijlstra LE, et al. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome. J Am Coll Cardiol. 2019;74(9):1167-1176. doi:10.1016/j.jacc.2019.03.013
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
- Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation. 2018;137(4):338-350. doi:10.1161/CIRCULATIONAHA.117.032235
- Charytan DM, Sabatine MS, Pedersen TR, et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J Am Coll Cardiol. 2019;73(23):2961-2970. doi:10.1016/j.jacc.2019.03.513
- Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618-628. doi:10.1016/S2213-8587(19)30158-5
- Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485-494. doi:10.1016/j.jacc.2014.02.615
- Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390:1962-1971. doi:10.1016/S0140-6736(17)32290-0
- Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633-643. doi:10.1056/NEJMoa1701131
- Rosoff DB, Bell AS, Jung J, Wagner J, Mavromatis LA, Lohoff FW. Mendelian randomization study of PCSK9 and HMG-CoA reductase inhibition and cognitive function. J Am Coll Cardiol. 2022;80(7):653-662. doi:10.1016/j.jacc.2022.05.041
- Amgen announces results from two open label extension studies of Repatha (evolocumab). News release. Amgen; April 27, 2022. Accessed July 6, 2022. www.amgen.com/newsroom/press-releases/2022/04/amgen-announces-results-from-two-open-label-extension-studies-of-repatha-evolocumab
- Boccara F, Kumar PN, Caramelli B, et al. Evolocumab in HIV-infected patients with dyslipidemia. J Am Coll Cardiol. 2020;75(20):2570-2584. doi:10.1016/j.jacc.2020.03.025
- Dayoub EJ, Eberly LA, Nathan AS, et al. Adoption of PCSK9 inhibitors among patients with atherosclerotic disease. J Am Heart Assoc. 2021;10(9):e019331. doi:10.1161/JAHA.120.019331
- Chamberlain AM, Gong Y, Shaw KM, et al. PCSK9 inhibitor use in the real world: data from the National Patient-Centered Research Network. J Am Heart Assoc. 2019;8(9):e011246. doi:10.1161/JAHA.118.011246
- Sumarsono A, Lalani HS, Vaduganathan M, et al. Trends in utilization and cost of low-density lipoprotein cholesterol–lowering therapies among Medicare beneficiaries: an analysis from the Medicare Part D database. JAMA Cardiol. 2021;6(1):92-96. doi:10.1001/jamacardio.2020.3723
- A Randomized Trial Assessing the Effects of Inclisiran on Clinical Outcomes Among People With Cardiovascular Disease (ORION-4). ClinicalTrials.gov. Published March 2, 2022. Accessed August 4, 2022. clinicaltrials.gov/ct2/show/NCT03705234
- Cardiovascular disease: a costly burden for America - projections through 2035. American Heart Association. Published 2017. Accessed July 6, 2022. www.heart.org/-/media/files/get-involved/advocacy/burden-report-consumer-report.pdf
- Smith CD, Balatbat C, Corbridge S, et al; American College of Physicians. Implementing optimal team-based care to reduce clinician burnout. NAM Perspect. 2018;8(9). doi:10.31478/201809c
- Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139. doi:10.1016/j.jacc.2015.09.025
- Dixon DL, Khaddage S, Bhagat S, Koenig RA, Salgado TM, Baker WL. Effect of pharmacist interventions on reducing low-density lipoprotein cholesterol (LDL-C) levels: a systematic review and meta-analysis. J Clin Lipidol. 2020;14(3):282-292.e4. doi:10.1016/j.jacl.2020.04.004
- Elkomos M, Jahromi R, Kelly MS. Pharmacist-led programs to increase statin prescribing: a narrative review of the literature. Pharmacy. 2022;10(1):13. doi:10.3390/pharmacy10010013
- Deshotels MR, Virani SS, Ballantyne CM. Lipid monitoring after initiation of lipid-lowering therapies: return of performance measures? Curr Cardiol Rep. 2021;23(9):116. doi:10.1007/s11886-021-01545-9
- Statin therapy for patients with cardiovascular disease and diabetes (SPC/SPD). National Committee for Quality Assurance (NCQA). Accessed August 18, 2022. www.ncqa.org/hedis/measures/statin-therapy-for-patients-with-cardiovascular-disease-and-diabetes/
- Jia X, Al Rifai M, Ramsey DJ, et al. Association between lipid testing and statin adherence in the Veterans Affairs Health System. Am J Med. 2019;132(9):e693-e700. doi:10.1016/j.amjmed.2019.04.002
- Rana JS, Virani SS, Moffet HH, et al. Association of low-density lipoprotein testing after an atherosclerotic cardiovascular event with subsequent statin adherence and intensification. Am J Med. 2022;135(5):603-606. doi:10.1016/j.amjmed.2021.11.011
- Lowenstern AM, Li S, Navar AM, et al. Measurement of low-density lipoprotein cholesterol levels in primary and secondary prevention patients: insights from the PALM Registry. J Am Heart Assoc. 2018;7(18). doi:10.1161/JAHA.118.009251
- Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206. doi:10.1001/jamacardio.2018.4936
- Tran C, Vo V, Taylor P, Koehn DA, Virani SS, Dixon DL. Adherence to lipid monitoring and its impact on treat intensification of LDL-C lowering therapies at an urban academic medical center. J Clin Lipidol. Published online May 2022:S1933287422000812. doi:10.1016/j.jacl.2022.05.003
- PCSK9 inhibitors for treatment of high cholesterol: effectiveness, value, and value- based price benchmarks. Institute for Clinical and Economic Review. Published November 24, 2015. Accessed July 6, 2022. icer.org/wp-content/uploads/2020/10/Final-Report-for-Posting-11-24-15-1.pdf
- Lin GA, Kazi DS, Jih J, Agboola F, Chapman R, Pearson SD. Inclisiran and bempedoic acid for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value; Evidence Report. Institute for Clinical and Economic Review. Published January 22, 2021. Accessed April 6, 2022. icer.org/wp-content/uploads/2021/02/ICER_High-Cholesterol_Evidence-Report_012221.pdf
- Desai NR, Campbell C, Electricwala B, et al. Cost effectiveness of inclisiran in atherosclerotic cardiovascular patients with elevated low-density lipoprotein cholesterol despite statin use: a threshold analysis. Am J Cardiovasc Drugs. Published online May 21, 2022. doi:10.1007/s40256-022-00534-9
- Study Evaluating Effectiveness and Adherence of Inclisiran Plus Standard of Care (SoC) Lipid-lowering Therapy Compared to SoC in ASCVD (VICTORION REAL). ClinicalTrials.gov. Published June 1, 2022. Accessed August 18, 2022. clinicaltrials.gov/ct2/show/NCT05399992
- Sharma SP, Russo A, Deering T, Fisher J, Lakkireddy D. Prior authorization. JACC Clin Electrophysiol. 2020;6(6):747-750. doi:10.1016/j.jacep.2020.04.022
- Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
- Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243-254. doi:10.1002/clc.22713
- Cohen JD, Cziraky MJ, Jacobson TA, Maki KC, Karalis DG. Barriers to PCSK9 inhibitor prescriptions for patients with high cardiovascular risk: results of a healthcare provider survey conducted by the National Lipid Association. J Clin Lipidol. 2017;11(4):891-900. doi:10.1016/j.jacl.2017.04.120
- Warden BA, Shapiro MD, Fazio S. The role of the clinical pharmacist in a preventive cardiology practice. Ann Pharmacother. 2019;53(12):1214-1219. doi:10.1177/1060028019864669
- Kaufman TM, Warden BA, Minnier J, et al. Application of PCSK9 inhibitors in practice: part 2: the patient experience. Circ Res. 2019;124(1):32-37. doi:10.1161/CIRCRESAHA.118.314191
- Michael O’Riordan. Pricey inclisiran is rolling out: a ‘buy-and-bill’ model may smooth its path. tctmd.com. Published January 17, 2022. Accessed April 6, 2022. www.tctmd.com/news/pricey-inclisiran-rolling-out-buy-and-bill-model-may-smooth-its-path
