- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
The Impact of Immune Checkpoint Inhibitors on Cost and Quality of Life in the Initial Treatment of Patients With Advanced or Metastatic NSCLC
Immune checkpoint inhibitors (ICIs) represent a significant benefit for the initial treatment of patients with advanced or metastatic non−small cell lung cancer (NSCLC). Beyond clinical benefit of increased overall survival, it represents a class of medications with a favorable adverse effect profile compared with chemotherapy. Drugs that target programmed cell death protein 1 (PD-1) receptor and its ligand PD-L1 are ICIs that work by taking the brakes off the immune system and promote T-cell−mediated cancer cell destruction. Current NCCN guidelines recommend that all patients with advanced or metastatic NSCLC have their PD-L1 status (amount of PD-L1 on cancer cells) assessed to guide treatment section. However, this recommendation is not always followed and may lead to inappropriate treatment selection and potential increased cost. Through appropriate biomarker testing, subsequent appropriate utilization of ICIs may help to drive down other costs and improve health-related quality of life. Managed care pharmacists should continue to focus on promotion of guideline concordant care that includes appropriate biomarker testing and selection of an evidence-based preferred treatment option.
Am J Manag Care. 2021;27(suppl 18):S333-S339. https://doi.org/10.37765/ajmc.2021.88770
Introduction
Lung cancer is the second most common type of cancer among men and women in the United States, accounting for approximately 12.4% of all new cancer cases. There are an estimated 235,760 new cases of lung cancer in 2021, along with an estimated 131,880 deaths (21.7% of all cancer deaths).1 Patients with non−small cell lung cancer (NSCLC) can present with either adenocarcinoma, squamous cell, or large cell carcinoma. Research has shown that NSCLC is a heterogeneous disease at the cellular and histologic level. These differences have a significant impact on pathogenesis, diagnosis, and selection of a targeted treatment regimen.2
A retrospective analysis of data from the Surveillance, Epidemiology, and End Results (SEER) database showed that mortality from NSCLC decreased at a faster rate than the incidence from 2013 to 2016. This decrease in mortality correlated to the approval of targeted therapy, which has been shown to significantly improve overall survival (OS) in this population. For men, the mortality rate for NSCLC declined by 3.2% annually from 2006 to 2013, and declined by a faster rate of 6.3% annually from 2013 to 2016. During this time, incidence decreased at an annual rate of 3.1% from 2008 to 2016. A similar increase in survival was seen in the female population as well. This study also noted that in the same timeframe, mortality in the small cell lung cancer population only decreased as a result of decreased incidence.3
The most recent advances in the initial treatment of advanced and metastatic NSCLC are immune checkpoint inhibitors (ICIs), a form of targeted immuno-oncology (IO). At this time there are 2 major targets for ICIs: cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) and its receptor PD-L1.4 Ipilimumab is a monoclonal antibody that binds to CTLA-4 and inhibits the negative regulator effect that it has on T-cell activity. This in turn has been shown to increase T-cell (eg, tumor infiltrating T-effector cells) activation and proliferation as well as decrease T-regulatory cell function. While effective, ipilimumab is not recommended as a preferred first-line agent for advanced or metastatic NSCLC.5
Another ICI target is PD-1 and its ligand PD-L1. The PD-1 checkpoint protein is found on T cells, and serves as an off switch to prevent cells from attacking each other. When PD-1 binds to its receptor PD-L1, it signals to the T cell to not attack. The problem arises when cancer cells exhibit PD-L1, and therefore escape T cell-mediated destruction. As of August 2021, the FDA had approved several therapies that target and bind to either PD-1, PD-L1, or CTLA-4 (pembrolizumab, nivolumab plus ipilimumab, cemiplimab, atezolizumab) for the first-line treatment of advanced or metastatic NSCLC. In general, selection of an agent is guided by the presence of a targeted treatment (eg, epidermal growth factor receptor status) identified through molecular testing. However, in patients who are PD-L1 positive with no other actionable molecular markers, regimens that include PD-1 and PD-L1 inhibitors are the preferred first-line agents for advanced or metastatic NSCLC.4,6 These recommendations are summarized in Table 1.6
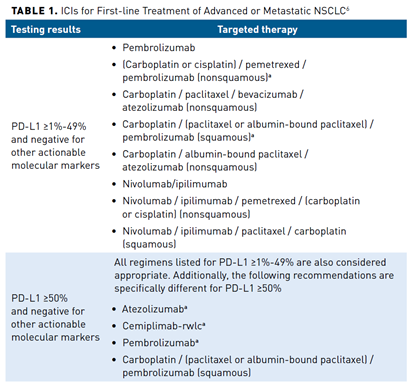
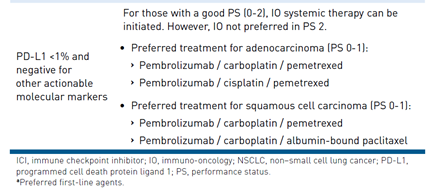
Determination of which agent to select is determined on the PD-L1 status.7 As summarized in Table 1,6 treatment of patients who express PD-L1 is segregated into 3 categories: (1) PD-L1 less than 1%, (2) PD-L1 greater than or equal to 1% to 49%, and (3) PD-L1 greater than or equal to 50%. Clinical trials have demonstrated OS benefit of regimens specifically in these PD-L1 ranges. It is recommended to test all patients with newly diagnosed advanced or metastatic NSCLC for PD-L1 status to select a treatment regimen with optimal OS benefit. However, there is not one standard assay for PD-L1 status and each ICI has a specific FDA-approved companion diagnostic immunohistochemistry assay. Table 28 summarizes the current ICIs recommended in first-line regimens for advanced or metastatic NSCLC and companion diagnostic immunohistochemistry assays.
Not all patients with advanced or metastatic NSCLC present with the same level of PD-L1 expression. In a worldwide, retrospective study of real-world evidence of 2617 adult patients (aged ≥18 years) with histologically confirmed (stage IIIB/IV NSCLC) found that 2368 (90%) of patients had PD-L1 data evaluated with PD-L1 IHC 22C3 pharmDx. Of this evaluable population, a majority had PD-L1 less than 1% (n = 1136, 48%), followed by PD-L1 greater than or equal to 1% to 49% (n = 702, 30%) and PD-L1 greater than or equal to 50% (n = 530, 22%).9
Economic Impact of Insufficient Biomarker Testing
Clinical trials have supported the OS benefit of biomarker testing to guide treatment selection based on PD-L1 status. This benefit is reflected in NCCN guidelines as a recommendation to test all patients with newly diagnosed advanced or metastatic NSCLC for PD-L1 status. However, real-world concordance with these recommendations is not always adhered to. While these tests all measure PD-L1 status, they use different antibody clones, staining platform, and scoring system (eg, tumor proportion score, tumor cell, tumor infiltrating immune cells). Therefore, specific tests need to accompany specific ICIs. Efforts have been taken to determine if these tests can be interchanged. In the Blueprint Phase 2 Project, study investigators suggested interchangeability between PD-L1 IHC 22C3 pharmDx, PD-L1 IHC 28-8 pharmDx, and VENTANA PD-L1(SP263) Assay, but not VENTANA PD-L1(SP142) Assay.10,11 However, use of an unapproved companion diagnostic test may lead to the inappropriate identification of a treatment eligible, or ineligible, patient.
One of the drawbacks of utilizing ICIs for the treatment of NSCLC is the limitation on testing for biomarkers. Insufficient or improper usage of biomarker testing may lead to underutilization of effective treatments, increased risk of inappropriate care, and increased unnecessary costs. In a retrospective observational claims study of the IBM Watson Health MarketScan Commercial and Medicare Supplemental Claims and Encounters databases from October 2012 to September 2017, a total of 3095 patients with advanced or metastatic NSCLC treated with an ICI were propensity matched to a population of 3095 patients with NSCLC who were eligible for ICI therapy but were treated with another regimen. Study investigators found that the overall utilization of PD-L1 testing was moderate at 0.6 and 0.7 tests per patient in the ICI treated and non-ICI treated group. This represents a significant discordance with current guidance to test all patients for PD-L1 status. Those in the ICI group incurred significantly higher total costs per patient than the non-ICI treated group in the 6 months post-treatment initiation ($141,537 vs $75,429; P <.0001). This study suggested that some patients who received ICI therapy did not have PD-L1 testing before treatment.12 This in turn could lead to inappropriate additional costs. For example, if a patient had a PD-L1 greater than or equal to 50% and negative for other actionable molecular markers, monotherapy with either atezolizumab, cemiplimab-rwlc, or pembrolizumab are preferred regimens for initial treatment. Inappropriate usage of treatment regimens may have incurred unnecessary direct costs from combination ICI plus chemotherapy treatment regimens and other costs such as treating chemotherapy-related adverse effects (AEs) versus immune-related adverse events (irAEs).13
AEs associated with chemotherapy are clinically and economically burdensome. In general, IOs (eg, ICIs) are considered safer as they are not directly cytotoxic. irAEs are associated with IO and can necessitate hospitalization, if severe. In a single center study, the cost associated with treating patients on ICI therapy from February 2011 to June 2017 was collected and summarized. While the annual cumulative costs due to irAEs increased from $218,700 to $1.3 million, the average cost per day of irAE hospitalization was significantly less than non-irAE oncology-related hospitalizations ($3142/day vs $8257/day; P = 10-10). Study investigators noted that this decrease in cost per day was possibly associated with irAEs being more readily intervenable.13
A systematic review of studies conducted between 2009 and 2019 evaluated the cost-effectiveness of biomarker tests for targeted therapy (including ICIs) compared with nonguided therapy or chemotherapy. Thirty-seven studies (conducted in multiple countries) were identified, with 64 different scenarios. Of those 64 scenarios, 34 scenarios (53%) were cost-effective, 28 (44%) were not cost-effective, and 2 were neither.14 Cost-effectiveness was dependent on an individual country’s willingness to pay threshold, which is usually set at 3 times that country’s gross domestic product per capita.15 When study authors compared biomarker-guided treatment selection to therapy for all patients approach, all scenarios were cost-effective. A limitation of this study was that a majority of studies (81%) were inappropriately designed for cost-effectiveness. However, all of the remaining 7 studies (19%) demonstrated cost-effectiveness of biomarker testing.14
Impact of ICIs on Quality of Life and Resource Utilization in the Initial Treatment of Advanced or Metastatic NSCLC
Atezolizumab
No studies were identified regarding the health-related quality of life (HRQOL) of atezolizumab monotherapy as initial treatment of advanced or metastatic NSCLC. However, a study that compared combination atezolizumab, bevacizumab, carboplatin, and paclitaxel to bevacizumab, carboplatin, and paclitaxel or atezolizumab, carboplatin, and paclitaxel had secondary end points that evaluated HRQOL on the Quality-of-Life Questionnaire Core 30 items (QLQ-C30) and Quality-of-Life Questionnaire Lung Cancer 13 items (QLQ-LC13) scales. No significant differences were observed between treatment groups regarding HRQOL measures.16
Pembrolizumab
One study evaluated the HRQOL of pembrolizumab monotherapy for the initial treatment of advanced PD-L1−positive NSCLC. The KEYNOTE-024 trial was a pivotal open-label phase 3, randomized trial that compared pembrolizumab to chemotherapy in patients with PD-L1 greater than or equal to 50%. The key prespecified exploratory end points were baseline-to-week-15 change in the QLQ-C30 (100-point scale) global health status (GHS)/quality-of-life (QOL) score, and the time to deterioration of the composite of cough, chest pain, and dyspnea in the QLQ-LC13. Pembrolizumab monotherapy showed a significantly better (lower) QLQ-C30 score at 15 weeks compared with chemotherapy with a difference of 7.8 (95% CI, 2.9-12.8; P = .0020). Additionally, time to deterioration was also longer in the pembrolizumab group compared with chemotherapy with a hazard ratio of 0.66 (95% CI, 0.44-0.97; P = .029). This study suggests that for patients with advanced or metastatic NSCLC and a PD-L1 greater than or equal to 50%, first-line treatment with pembrolizumab provides an HRQOL benefit over chemotherapy.17
This benefit in HRQOL of pembrolizumab was also demonstrated in combination with chemotherapy compared with chemotherapy alone in patients with nonsquamous metastatic NSCLC. In the KEYNOTE-189 study, pembrolizumab plus pemetrexed–platinum had better maintained GHS/QOL at week 21 compared with placebo plus pemetrexed–platinum with a between-group difference of 5.3 points (95% CI, 1.1-9.5); P = .014). Additionally, there was no significant difference in the time to deterioration between groups.18 Patients in this study had presented with varied PD-LQ status of PD-L1 less than 1% (31%), PD-L1 greater than or equal to 1% to 49% (31.2%), and a PD-L1 greater than or equal to 50% (32.2%).19 These findings further support the HRQOL benefit of pembrolizumab added to pemetrexed–platinum for patients of varied PD-L1 status. An argument can also be made that a more pronounced benefit may have been observed if the PD-L1 status was limited to greater than or equal to 50%.18
Nivolumab
The CheckMate 227 study was an open-label trial that evaluated nivolumab plus ipilimumab compared with chemotherapy for the initial treatment of advanced NSCLC with a high tumor burden. Beyond efficacy and safety, one of the exploratory end points was Lung Cancer Symptom Scale LCSS 3-item global index (LCSS 3-IGI), which was on a 300-point scale (higher number, better HRQOL). In this study, a minimally important difference was noted as a change in score of 30 or more. At week 6, there were noted improvements in HRQOL in the nivolumab plus ipilimumab arm compared with the chemotherapy arm that reached a minimally important difference at 12 weeks that was maintained through the rest of the study.20
Cemiplimab
The EMPOWER-Lung 1 trial was a randomized, open-label, multicenter, phase 3 study that evaluated cemiplimab dosed 350 mg intravenously every 3 weeks (up to 108 weeks) or 4 to 6 cycles of provider’s choice platinum-doublet therapy for initial treatment of advanced NSCLC with PD-L1 greater than or equal to 50%. The median follow-up time in this study was about 10.8 months in both groups. In regard to efficacy, median overall survival was not reached in the cemiplimab group (n = 283), and was 14.2 months in the chemotherapy group (n = 280). This translated to a significantly reduced risk for those in the cemiplimab group compared with chemotherapy with a hazard ratio of 0.57 (95% CI, 11.2-17.5; P = .0002). Median progression-free survival was also significantly higher in the cemiplimab group (8.2 months) compared with the chemotherapy group (5.7 months), with a hazard ratio for disease progression of 0.54 (95% CI, 0.43-0.68; P <.001). Utilizing the intention-to-treat population, which included some patients with lower PD-LI expression, fewer patients in the cemiplimab group (n = 356) versus the chemotherapy group (n = 354) had grade 3-4 treatment-emergent AEs (28% vs 39%, respectively). QOL was also assessed as a secondary outcome in the intention-to-treat population, and a meaningful change in QLQ-C30 from baseline throughout the trial was observed in only patients on cemiplimab, not chemotherapy. While not definitive, this may have been attributed to the favorable benefit and AE profile of cemiplimab compared with chemotherapy and/or the open-label design of the study.21
Other QOL Considerations
A real-world patient-reported outcome study of patients who received IO or chemotherapy plus IO for metastatic NSCLC was evaluated to determine if clinical practice differed significantly from clinical trials. From 2017 to 2018, patients at a single academic medical center completed the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC-QLQ-C30) and the National Cancer Institute Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). A total of 60 patients participated, of which 57% received a single-agent IO. Results of this study showed a mean EORTC-QLQ-C30 global health score of 62.6, which was similar to second-line single-agent IO (65-67), but worse than first-line IO with pembrolizumab alone (71). Of note, insomnia and financial concerns were meaningfully worse than what was observed in previous trials. The most common concerns noted also included dermatologic symptoms, cough, arthralgia, and myalgia. Study investigators note that a limitation of this study may be that the sampled population may have been at a lower socioeconomic status or had more noncancer comorbidities. Results suggest that healthcare providers should expect similar QOL and symptom burden as seen in clinical trials, but real-world factors may adversely affect these values.22
With the increased OS seen in patients on IO-based treatment regimens, some patients are living beyond their initial life expectancy. In one international study of 24 survivors who received IO for stage IV NSCLC, qualitative interviews and other assessments were performed by study investigators who spent a total of 2 days at their homes and health-related outings. Investigators noted that long-term survivors of patients who were on IO experienced their journey in 2 phases. The first phase was when their cancer diagnosis took over all aspects of their life. The second phase occurred when cancer only took a small part of everyday living. Those patients who lived beyond the initial prognosis of a terminal disease lived in what was described as a limbo period, where life returned almost to normal besides having a terminal diagnosis. This limbo period impacted various aspects of the survivor’s life that included life priorities, decision making, how they experienced patient support, and how they sought health information. All of these changes were noted to impact QOL in a way that may not be well understood by healthcare providers, the cancer community, or by current QOL assessment tools that were designed before IO treatment options were available. What investigators recommended was increased awareness of healthcare providers on how increased life expectancy may change a patient’s view on QOL and how to best direct resources to the patient.23
Resource Utilization
The treatment of advanced and metastatic NSCLC continues to be a high economic burden. High costs of care are driven by inpatient, outpatient, and medication costs associated with treatment. While varying studies have supported the cost-effectiveness of ICIs in the initial treatment of advanced or metastatic NSCLC, treatment can be expensive due to high direct costs of care.24 Previous studies have evaluated the total cost of care and resource usage. However, many have not accounted for the added mortality benefit, improved safety profile, and other considerations of ICI treatment. There still remain limited studies that have evaluated the resource utilization of ICIs in the initial treatment of advanced and metastatic NSCLC.25
Previously discussed was the potential for decreased usage of hospital resources for managing treatment-emergent AEs or irAEs. One real-world claims-based study found that AEs were least common in patients treated with IO compared with either chemotherapy or IO plus chemotherapy with an incident rate ratio of 1.4 for both. This can be interpreted as a 40% higher relative rate of AEs in the chemotherapy or IO plus chemotherapy group compared with IO alone. This translated to a significantly lower per-member per-month cost of AEs in the IO cohort ($4259) compared with either chemotherapy ($6323; P <.001) or IO plus chemotherapy ($6269; P = .020).26
A single-center retrospective chart review of STAnford medicine Research data Repository (STARR) data indicated that 97 patients with NSCLC treated with either a tyrosine kinase inhibitor (TKI), ICI-based regimen, or chemotherapy visited the emergency department (ED) in 2018 a total of 173 times. Therapy-related encounters were a significantly smaller portion of ED visits in the TKI group (2%), ICI group (12%), and chemotherapy group (21%). This real-world evidence supports the potential for decreased healthcare resource utilization associated with ICIs compared with chemotherapy.27 For patients receiving ICIs, providers should closely monitor for AEs that are expected to be immune related to prevent additional healthcare costs.
A retrospective claims review of Medicare Part D patients in the Optum Research Database from March 1, 2014, through April 30, 2019, evaluated the healthcare resource utilization, cost, and mortality of those who received a PD-1− or PD-L1−targeted ICI. Results showed that patients who had an AE associated with ICI therapy were at a 2-fold greater risk of an inpatient hospital stay with a hazard ratio of 2.2 (95% CI, 1.9-2.5) and a higher risk of an emergency visit with a hazard ratio of 1.8 (95% CI, 1.6-2.1) than patients who did not experience an AE. This translated to a significantly higher adjusted 6-month cost of $24,301 (95% CI, $18,828-$29,774; P <.001). The main driver for this increased cost was associated with inpatient visits that accounted for 89% of the total. Mortality was noted as similar between groups that had or did not have an AE.28
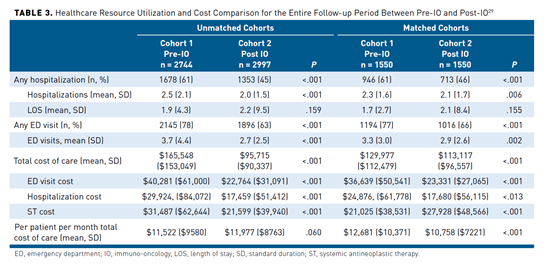
To better understand the impact of IO on the total cost of care associated with the initial treatment of advanced NSCLC, one study was conducted that assessed the direct costs between a pre-immuno-oncology (pre-IO) period from March 2013 to March 2014 to a post-immuno-oncology (post-IO) period from March 2015 to December 2016. Patients were identified through the Medical Outcomes Research for Effectiveness and Economics RegistryResearch database, which is composed of multiple payer data. A total of 5741 patient records were included, and were propensity score matched to include 1550 in each cohort. Results from the study show that in the matched cohorts, there were significantly fewer hospitalizations (61% vs 46%; P <.001), any ED visit (77% vs 66%; P <.001), mean number of ED visits (3.3 vs 2.9; P <.002), total cost of care ($129,977 vs $113,117; P <.001), and mean per-patient per-month total cost of care ($12,681 vs $10,758; P <.001). These results are summarized in Table 3.29

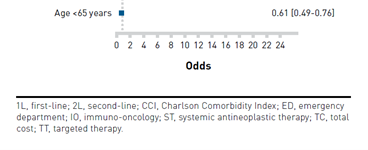
Compared with second-line or third-line treatment, only the first-line treatment mean total cost of care was only significantly higher in the pre-IO group compared with the post-IO group ($87,890 vs $80,206; P = .011). Additionally, when taking a look at predictors of a higher total cost in the post-IO group, the variables that stand out include use of IO in any line of treatment, first-line combination therapy, and biomarker testing. A limitation of this study was that a characterization of what chemotherapy or IO was not provided in the pre-IO and post-IO periods. A breakdown of these variables is illustrated in the Figure.29
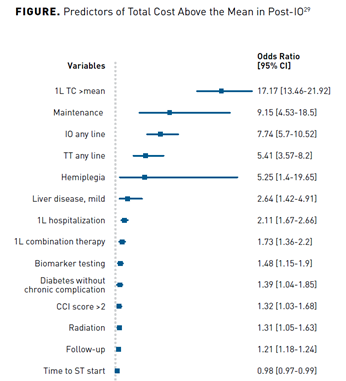
Conclusions
The initial management of advanced or metastatic NSCLC has evolved with the incorporation of ICIs into the treatment paradigm. These therapies have not only demonstrated a significant increase in OS, but have also provided a treatment option with potentially fewer AEs. One limitation regarding the utility of ICIs for treatment is the need for specific biomarker companion assays to help determine appropriate treatment selection. At this time, these assays are not interchangeable, and companion tests have to be used with their corresponding companion ICI. Current NCCN guidelines recommend that all patients with advanced or metastatic NSCLC have PD-L1 status assessed with an FDA-approved test to determine status. However, real-world concordance with this recommendation is not 100%. Patients with PD-L1 greater than or equal to 50% are able to start monotherapy with an ICI that includes atezolizumab, cemiplimab-rwlc, or pembrolizumab. Real-world evidence has shown that use of IO may result in lower healthcare resource utilization, fewer ED visits, and potentially lower costs. Additionally, IOs may offer a more favorable benefit/risk profile compared with chemotherapy that may be associated with decreased AEs and cost. The use of ICIs for initial treatment of advanced or metastatic NSCLC is also supported through increased HRQOL, especially in the PD-L1 greater than or equal to 50% population treated with pembrolizumab. As patients continue to live beyond the initial prognosis, it is important for healthcare providers to assess and discuss with their patients what is important to them now and how their healthcare should be prioritized. Managed care pharmacists should prioritize guideline concordance to biomarker testing and utilization of preferred treatment options that can increase HRQOL while controlling costs. Clinical treatment pathways may be considered to help guide optimal treatment selection guided by preferred ICI-based treatment regimens.
Author affiliation: Kelly Procailo, PharmD, BCOP, is manager, Oncology Medical Drug Management and Customer Initiatives, Blue Cross Blue Shield of Michigan, Detroit, MI.
Funding source: This activity is supported by educational grants from BeiGene, Ltd. and Merck Sharp & Dohme Corp.
Author disclosure: Dr Procailo has no relevant financial relationships with commercial interests to disclose.
Author information: Concept and design; drafting of the manuscript; and critical revision of the manuscript for important intellectual content.
Address correspondence to: kprocailo@bcbsm.com
Medical writing and editorial support provided by: Andrew Abe, PharmD
References
1. Cancer of the lung and bronchus - Cancer Stat Facts. Surveillance, Epidemiology, and End Results (SEER). Accessed August 6, 2021. https://seer.cancer.gov/statfacts/html/lungb.html
2. de Sousa VM, Carvalho L. Heterogeneity in lung cancer. Pathobiology. 2018;85(1-2):96-107. doi:10.1159/000487440
3. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi:10.1056/NEJMoa1916623
4. American Cancer Society. Immune checkpoint inhibitors and their side effects. Accessed August 9, 2021. www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html
5. Yervoy. Prescribing information. Bristol Myers Squibb; May 2021. Accessed September 6, 2021. https://packageinserts.bms.com/pi/pi_yervoy.pdf
6. National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2021). June 15, 2021. Accessed August 7, 2021. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
7. Theelen WS, Baas P. Pembrolizumab monotherapy for PD-L1 ≥50% non-small cell lung cancer, undisputed first choice? Ann Transl Med. 2019;7(suppl 3):S140. doi:10.21037/atm.2019.06.35
8. Health C for D and R. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). FDA. Updated September 2, 2021. Accessed September 6, 2021. www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools
9. Dietel M, Savelov N, Salanova R, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174-179. doi:10.1016/j.lungcan.2019.06.012
10. Velcheti V, Patwardhan PD, Liu FX, Chen X, Cao X, Burke T. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS One. 2018;13(11):e0206370. doi:10.1371/journal.pone.0206370
11. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint Phase 2 Project. J Thorac Oncol. 2018;13(9):1302-1311. doi:10.1016/j.jtho.2018.05.013
12. Nesline MK, Knight T, Colman S, Patel K. Economic burden of checkpoint inhibitor immunotherapy for the treatment of non–small cell lung cancer in US clinical practice. Clin Ther. 2020;42(9):1682-1698.e7. doi:10.1016/j.clinthera.2020.06.018
13. Chu JN, Choi JG, Ostvar S, et al. Cost of inpatient admissions for immune-related adverse effects from immune checkpoint inhibitor therapy: a single center experience. J Clin Oncol. 2018;36(15_suppl):3060-3060. doi:10.1200/JCO.2018.36.15_suppl.3060
14. Henderson R, Keeling P, French D, Smart D, Sullivan R, Lawler M. Cost-effectiveness of precision diagnostic testing for precision medicine approaches against non-small-cell lung cancer: a systematic review. Mol Oncol. Published online June 10, 2021. doi:10.1002/1878-0261.13038
15. Griffiths M, Maruszczak M, Kusel J. The WHO-choice cost-effectiveness threshold: a country-level analysis of changes over time. Value Health. 2015;18(3):A88. doi:10.1016/j.jval.2015.03.517
16. Reck M, Wehler T, Orlandi F, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without bevacizumab versus bevacizumab plus chemotherapy in non–small-cell lung cancer. J Clin Oncol. 2020;38(22):2530-2542. doi:10.1200/JCO.19.03158
17. Brahmer JR, Rodríguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600-1609. doi:10.1016/S1470-2045(17)30690-3
18. Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(3):387-397. doi:10.1016/S1470-2045(19)30801-0
19. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi:10.1056/NEJMoa1801005
20. Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137-147. doi:10.1016/j.ejca.2019.05.008
21. Sezer A, Kilickap S, Gümüs¸ M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592-604. doi:10.1016/S0140-6736(21)00228-2
22. McLouth LE, Lycan TW, Levine BJ, et al. Patient-reported outcomes from patients receiving immunotherapy or chemo-immunotherapy for metastatic non-small cell lung cancer in clinical practice. Clin Lung Cancer. 2020;21(3):255-263.e4. doi:10.1016/j.cllc.2019.11.015
23. Park R, Shaw JW, Korn A, McAuliffe J. The value of immunotherapy for survivors of stage IV non-small cell lung cancer: patient perspectives on quality of life. J Cancer Surviv. 2020;14(3):363-376. doi:10.1007/s11764-020-00853-3
24. Runyan A, Banks J, Bruni DS. Current and future oncology management in the United States. J Manag Care Spec Pharm. 2019;25(2):272-281. doi:10.18553/jmcp.2019.25.2.272
25. Lee DH, Isobe H, Wirtz H, et al. Health care resource use among patients with advanced non-small cell lung cancer: the PIvOTAL retrospective observational study. BMC Health Serv Res. 2018;18:147. doi:10.1186/s12913-018-2946-8
26. Engel-Nitz NM, Johnson MP, Bunner SH, Ryan KJ. Real-world costs of adverse events in first-line treatment of metastatic non-small cell lung cancer. J Manag Care Spec Pharm. 2020;26(6):729-740. doi:10.18553/jmcp.2020.26.6.729
27. Shah MP, Neal JW. Relative impact of anticancer therapy on unplanned hospital care in patients with non–small-cell lung cancer. JCO Oncol Pract. 2021;17(8):e1131-e1138. doi:10.1200/OP.20.00612
28. George S, Bell EJ, Zheng Y, et al. The impact of adverse events on health care resource utilization, costs, and mortality among patients treated with immune checkpoint inhibitors. Oncologist. 2021;26(7):e1205-e1215. doi:10.1002/onco.13812
29. Korytowsky B, Radtchenko J, Nwokeji ED, Tuell KW, Kish JK, Feinberg BA. Understanding total cost of care in advanced non-small cell lung cancer pre- and postapproval of immuno-oncology therapies. Am J Manag Care. 2018;24(20 suppl):S439-S447.
