- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
The Emerging Landscape of Alternative Pathway Complement Inhibitors
Dysregulation of the complement system can induce or exacerbate a wide range of disorders—from age-related macular degeneration (AMD) to rare, complement-mediated renal diseases and prothrombotic hematologic disorders—and contributes to excessive inflammation and tissue damage.1-3 The current landscape of approved therapeutics targeting the complement system involves inhibitors of the classical and terminal pathways.4-8 Alternative pathway-targeting therapeutics explore novel means of treating many complement-mediated diseases.9-20 Some of these agents are highly selective for the alternative pathway, leaving the classical and lectin pathways intact; this could result in a lower infection risk compared with use of other complement inhibitors.4-6,9,19,21 This article reviews the current landscape of therapeutics targeting the complement system and the emerging drugs targeting the alternative pathway to treat a wide range of diseases.
The Role of the Complement System
The complement system is a set of proteins that plays a central role in innate immunity, adaptive immune responses, and normal tissue homeostasis.1,22 The classical, lectin, and alternative pathways activate the complement system via distinct mechanisms.1 Upon activation on certain plasma and cell surfaces following recognition of danger signals, the complement system proteins are cleaved in a cascade-like fashion into bioactive components.1,22 These actions ultimately lead to the direct lysis of pathogens, pathogen phagocytosis by immune cells, and assembly and stimulation of inflammatory cells.1 Regulation of adaptive cellular immune responses and clearance of dead/dying cells and immune complexes from circulation are also functions of the complement system.1 The ability to distinguish pathogens from host cells and control unwanted complement activation against the host are key to the regulation and homeostasis of the complement system.1
Impact of Complement System Dysregulation
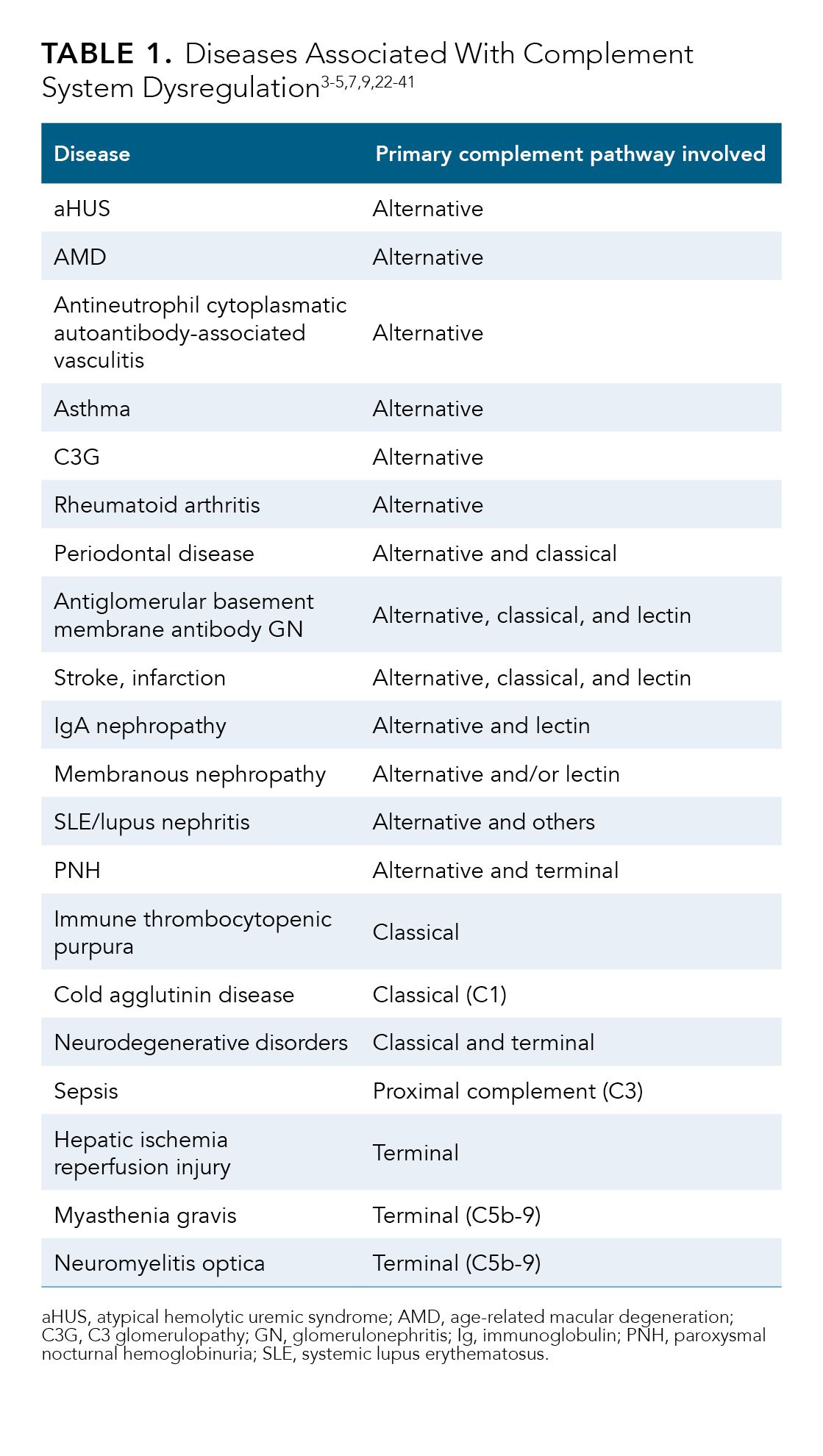
A network of proteins acts to regulate the complement system and protect the host from complement-mediated tissue damage.1 Dysregulation of the complement system contributes to excessive inflammation and tissue damage and induces or exacerbates a wide range of disorders including various chronic renal disorders, prothrombotic hematologic diseases, and ocular conditions.1,2 Table 1 lists many of the diseases and conditions in which the pathogenic role of the complement system has been at least partially elucidated in human or animal studies.1-5,7,9,22-41
Complement system dysregulation results from a deficiency or overactivation of 1 or more regulatory proteins.1,2 Regulatory protein deficiencies can be genetic or acquired.1,2,25,42 Adverse activation of the complement system can occur following exposure to high levels of a pathogen, damage-associated stimuli, or foreign surfaces.2 Uncontrolled conditions (eg, ischemia/reperfusion, sepsis, neurodegenerative disorders) can overwhelm regulatory proteins, resulting in excessive inflammation and complement-mediated tissue damage.1
The Role of the Alternative Pathway
Identification of immune complexes and pattern recognition signals trigger the classical and lectin pathways, respectively; however, under normal physiological conditions, the alternative pathway is the dominant complement surveillance method.22,43 The alternative pathway activates the complement system via the tick-over mechanism, in which low levels of complement protein 3 (C3) are constantly activated into C3b, which can deposit on any surface, ensuring prompt detection of pathogens or irritants.22,43 The classical, lectin, and alternative pathways converge at the level of the C3 convertase, with C3 being a central component to the complement system (Figure).22,43,44
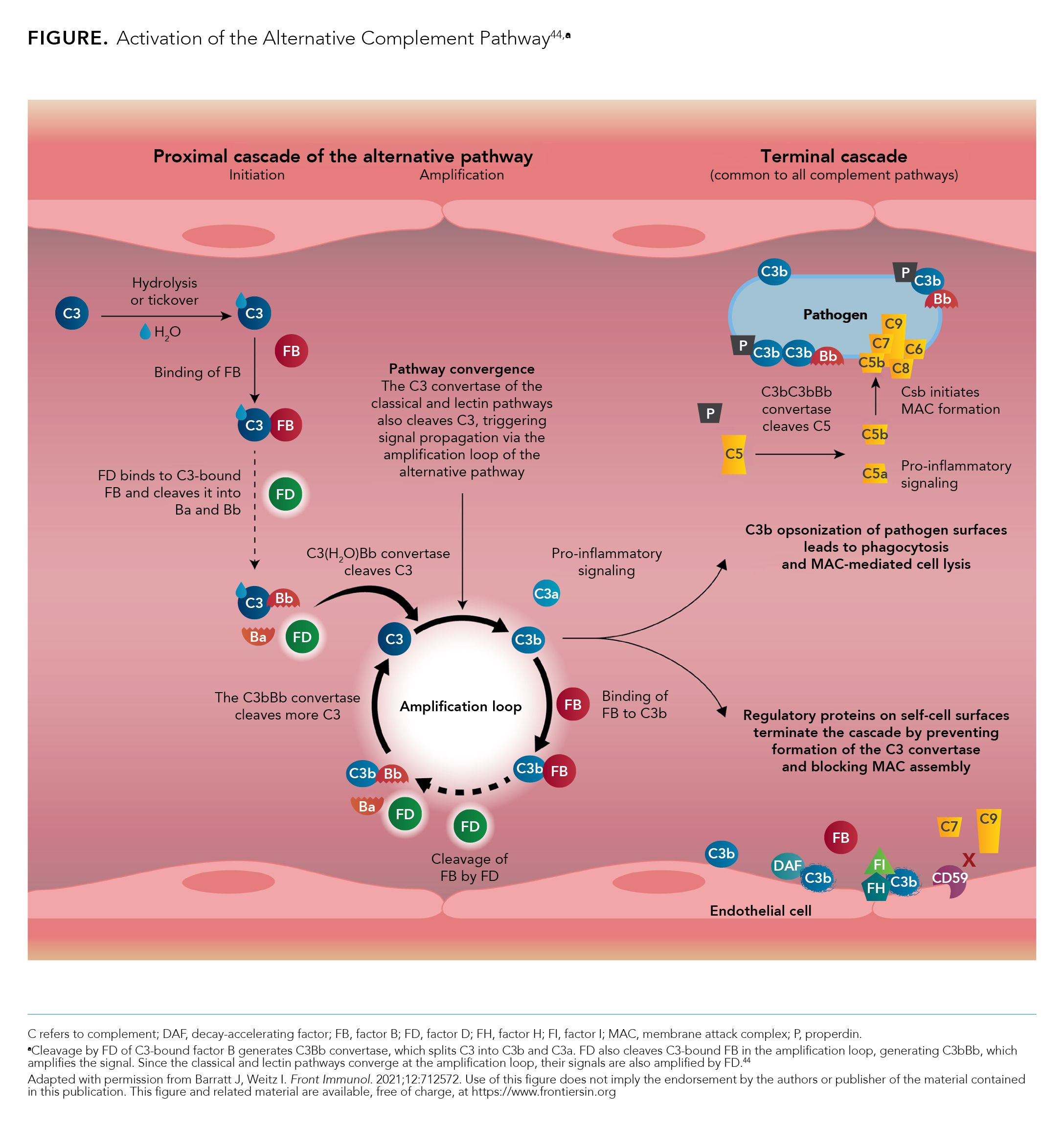
Under normal physiological conditions, C3b is inactivated by complement regulators, preventing damage to healthy host tissue.22 Complement regulators are mostly absent from pathogens and cell debris; when C3b binds to pathogens and cell debris, the activation of C3 will amplify as a positive feedback loop, with the alternative pathway C3 convertase C3bBb being generated by factors B, D, and properdin.22 The alternative pathway is key to the amplification loop of the complement system, as any C3b deposited by 1 of the 3 pathways can activate C3bBb of the alternative pathway.1 Factors B, D, and properdin are unique to the alternative pathway, with each playing a role in its activation and subsequent complement system amplification.22,43,45 Additional regulators (eg, factors H and I) also play a role in complement amplification.43
Burden of Alternative Pathway Dysregulation
Given the key role of the alternative pathway in the amplification of the complement system, its dysregulation has been implicated in several diseases, including AMD, C3 glomerulopathy (C3G), atypical hemolytic uremic syndrome (aHUS), and antineutrophil cytoplasmatic autoantibody-associated vasculitis.1,3,23,25,29 The alternative pathway is also an important contributor to the pathogenesis of conditions such as paroxysmal nocturnal hemoglobinuria (PNH), immunoglobulin (Ig) A nephropathy, lupus nephritis, membranous nephropathy, and antiglomerular basement membrane glomerulonephritis.9,22,24,26-28,30,31 Involvement of the alternative pathway has also been associated with conditions such as rheumatoid arthritis, asthma, ischemic stroke, and periodontal disease.32,33,36,37
AMD
AMD is a disorder of the macula distinguished by drusen (yellow lesions at the basement membrane level of the retinal pigment epithelium [RPE]) or other RPE alterations.46 Abnormalities of the RPE may include hypopigmentation, hyperpigmentation, or geographic atrophy, which is an advanced form of AMD involving the center of the fovea.46 Activation of the alternative complement pathway has been implicated in the pathogenesis of AMD, with levels of complement proteins (eg, C3, factors B and D) increasing with disease progression.23
AMD affects an estimated 1.75 million US adults 40 years and older.46,47 Patient visual acuity may range from normal to severely impaired.46 The results of 2 observational studies of patients with geographic atrophy secondary to AMD demonstrated that the lesion area caused by geographic atrophy substantially increased and visual function deteriorated over a 2-year period.48 AMD is associated with a considerable economic burden in the United States; annual direct medical costs have been estimated to be $575 million (2004 US$).49 Additionally, because of the accompanying visual impairment, patients are at risk for falls and fractures, which can add to the cost of care.50,51
PNH
PNH is a rare, acquired hematopoietic stem cell disorder characterized by chronic hemolysis.24,52 Somatic mutations in the PIGA gene lead to deficiencies in the complement inhibitory proteins CD55 and CD59, resulting in chronic complement-mediated hemolysis of affected erythrocytes.24 The alternative pathway, with its constant low-level activation state, contributes to the chronic nature of the hemolysis.24 Intravascular hemolysis resulting in moderate to severe anemia is a common clinical manifestation.24 In this setting, anemia also may be a result of bone marrow failure.24 Extravascular hemolysis of erythrocytes that survived intravascular hemolysis may also be present in patients treated with complement 5 (C5) inhibitors.24 Thrombosis, particularly venous thrombosis, also may occur in patients with PNH.24 Fatigue, dyspnea, hemoglobinuria, and abdominal pain are common symptoms of PNH.53
PNH is associated with considerable health care resource utilization (HCRU) and high costs, particularly among the many patients who depend upon red blood cell (RBC) transfusions.53,54 An analysis of 2014-2019 HCRU and cost data from IBM MarketScan Research Databases found that, among 151 patients 12 years and older being treated for PNH with eculizumab, those who were transfusion dependent had significantly higher all-cause direct medical costs than did those who were not. The adjusted per patient per year cost difference between those with 1 or more claims for blood transfusion within 6 months on or after the eculizumab treatment date (n = 55) and those who had no such claims (n = 96) was $247,848 (95% CI: $87,350-$445,161; P = .004) (2020 US$).54
Renal Effects
IgA nephropathy, a leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD), is the most common form of primary glomerular disease globally.25 In the United States, the incidence rate has been reported to be up to 2.3 per 100,000 persons/year.55 The underlying pathogenesis of IgA nephropathy is thought to involve complement activation leading to the formation of pathogenic immune complexes that result in mesangial deposition and glomerular injury.26 Factor H and activation of the lectin and alternative pathways also occur.26 The disease usually follows a slow, progressive course, with about 30% of affected patients developing ESRD within 25 years of initial presentation. Current management of IgA nephropathy focuses on supportive care to slow disease progression.25 More than half of affected patients in the United States have stage 3 or higher CKD at the time of diagnosis.55 Although limited information is available on the economic burden associated with IgA nephropathy in the United States, the US Centers for Disease Control and Prevention found that Medicare costs for patients with CKD were $87.2 billion in 2019; Medicare costs for patients with ESRD totaled an additional $37.3 billion.55,56
C3G
C3G is a rare, complement-mediated, membranoproliferative glomerulonephritis characterized by C3-dominant fragment deposition resulting from alternate pathway dysregulation.3,25 Patients may present with hematuria, kidney insufficiency, and a variable degree of proteinuria.57 Renal outcomes are often poor; results from a study of renal biopsies archived at the University of Utah Department of Pathology from 2005 to 2015 showed that, of 9 patients with C3G (age, ≥ 49 years) who had follow-up of at least 1 year, about half (n = 4) developed ESRD requiring chronic hemodialysis. Disease recurrence following kidney transplantation was also reported in 1 patient.57 The optimal treatment strategy for patients with C3G remains unclear, with current management focusing on supportive measures, and, in the case of moderate to severe disease, immunosuppression.25
Membranous Nephropathy
Membranous nephropathy is a kidney disorder associated with nephrotic syndrome, often caused by antibodies against the M-type phospholipase A2 receptor.25 The resulting subepithelial immunoglobulin deposits and activation of complement via the lectin and/or alternative pathway lead to injury of the glomerular basement membrane.9 Although spontaneous remission of membranous nephropathy may occur, some patients are at risk of progressive kidney function loss that results in ESRD.25,58 Supportive therapy is the primary treatment for patients with membranous nephropathy and proteinuria, whereas immunosuppressive therapy is reserved for those at risk of progressive kidney injury.25
In a 2019 study of 2012-2015 US commercial health insurance claims data for patients with idiopathic membranous nephropathy (N = 2689), 16% of patients had ESRD, 10% underwent dialysis, and 3.5% received a kidney transplant.58 Costs associated with dialysis and kidney transplant (ie, outpatient hospital use and ESRD facility use) were the primary drivers of the HCRU differences seen between the 5% of patients who constituted the high-cost cohort (HCC) and the remaining 95% of patients (non high–cost cohort [NHCC]).58 The HCC incurred 43.7% of total 1-year cost for all patients, which included hospitalization, emergency department, pharmacy prescription, and outpatient (outpatient hospital use, ESRD facility use, and office visits) costs paid by the patient and the commercial insurance plan. Mean per-patient 1-year costs for the HCC and NHCC were $401,608 and $27,154, respectively.58
aHUS
aHUS is a very rare form of thrombotic microangiopathy characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury resulting from endothelial cell damage involving the kidney vasculature.25,42 The disease is often caused by genetic or acquired functional defects in complement regulatory proteins that lead to dysregulation of the alternative complement pathway at the surface of endothelial cells.25,42 Prior to the introduction of complement inhibitors, the prognosis for patients with aHUS was poor, with most developing ESRD within 2 years of diagnosis.42 Current complement inhibitors offer the potential to prevent the progressive loss of kidney function, yet the clinical and treatment-related burdens associated with the disease remain substantial.42,59,60
A 2022 modeling study estimated treatment costs for a population of patients in the United States with aHUS who were treated with the C5 complement inhibitors eculizumab or ravulizumab (n = 100). Results of the analysis demonstrated that annualized discounted treatment costs over a lifetime for adults and their caregivers, including lost productivity costs, could exceed $210,000 (2020 US$). Limitations of the study included its assumptions regarding patient characteristics, treatment patterns, travel times, and level of caregiver support.60
Lupus Nephritis
Lupus nephritis is an immune complex-mediated glomerulonephritis that can develop in patients with the autoimmune disorder systemic lupus erythematosus (SLE).25 Activation of different components of the complement system, including the alternative pathway, are thought to occur in lupus nephritis.22,27,28 The prevalence of lupus nephritis among patients with SLE varies depending upon disease severity, with 1 study reporting rates of 8% for patients with mild SLE and 34% for patients with moderate/severe SLE between 2009 and 2016.61 The prevalence of ESRD among patients with lupus nephritis was 10.5% in a retrospective study of 2007-2019 Optum medical and pharmacy claims data for patients who received a diagnosis of SLE, had evidence of lupus nephritis, and had and at least 1 inpatient admission or at least 2 outpatient admissions separated by at least 30 days between January 2015 and December 2019 (N = 21,251). Additionally, all-cause medical costs for patients with lupus nephritis almost doubled during active disease periods (ie, during relapses or flares) compared with low disease–activity periods (mean, $4777/month vs mean, $2523/month; 2020 US$).62 Patients with ESRD had a mean monthly all-cause medical cost of $18,084, mainly driven by inpatient visit costs.62
Myasthenia Gravis
Myasthenia gravis is a neuromuscular disorder in which an abnormal autoimmune, antibody-mediated response affects the neuromuscular junction, resulting in muscle weakness and fatigue.63 Deposition of the terminal complement complex C5b-9, also known as the membrane attack complex, at the neuromuscular junction is thought to play a role in the pathogenesis of generalized myasthenia gravis.4,5 In 2015, the prevalence of myasthenia gravis in the United States was estimated to be 14 to 20 cases per 100,000 people; the disorder remains underdiagnosed, however, and the prevalence is likely higher.64 Patients with myasthenia gravis often report ocular involvement, ptosis, general fatigue, and weakness of arms, legs, hands, or fingers.65 Many patients report moderate to severe symptoms despite receiving chronic treatment.65
A study of Symphony Health Integrated Dataverse data identified US adults with at least 2 medical and/or pharmaceutical claims associated with myasthenia gravis between January 2014 and December 2019 (N = 41,490). Cost analysis of HCRU related to these claims estimated that patients with generalized myasthenia gravis with exacerbation events (N = 4355) had standardized 12-month mean all-cause (2018 US$) costs of $43,734 and direct costs for disease treatments of $21,550.66
Current Treatment Landscape and Unmet Needs
The current landscape of approved therapeutics targeting the complement system is dominated by inhibitors of the classical and terminal pathways.4-8 In 2007, eculizumab became the first complement inhibitor to receive FDA approval.67 A second complement inhibitor, ravulizumab, was approved in 2018 and a few other agents have been approved more recently.5-8 In addition, several other drugs targeting the classical and terminal pathways are being developed.22,68,69
Eculizumab and ravulizumab are injectable terminal complement inhibitors that block the activity of C5, preventing the formation of the C5b-9 terminal complement complex.4,5 These agents inhibit intravascular hemolysis mediated by the terminal complement in patients with PNH and complement-mediated thrombotic microangiopathy in patients with aHUS.4,5 Both agents also are approved for the treatment of adults with generalized myasthenia gravis who harbor antiacetylcholine receptor antibodies.4,5 Eculizumab also is approved for the treatment of adults with neuromyelitis optica spectrum disorder who harbor anti–aquaporin-4 antibodies.4 Both agents are under investigation as treatments for patients with one of several other diseases that may involve complement dysregulation including hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome (eculizumab) and dermatomyositis (ravulizumab).70,71
Pegcetacoplan is an injectable C3 inhibitor approved for the treatment of adults with PNH.6 This agent acts proximally in the complement system, regulating both C3b-mediated extravascular hemolysis and terminal complement–mediated intravascular hemolysis in PNH.6 In a phase 3 trial (NCT03500549), pegcetacoplan led to superior hemoglobin (Hb) level improvements compared with eculizumab in patients with PNH and baseline Hb levels less than 10.5 g/dL despite treatment with eculizumab.72 This agent is currently under investigation as treatment for patients with geographic atrophy secondary to AMD, cold agglutinin disease, and other diseases.73
Sutimlimab is an injectable classical complement pathway inhibitor that blocks the activity of C1, inhibiting hemolysis in patients with cold agglutinin disease.7 This agent was approved in 2022 to decrease RBC transfusion requirements due to hemolysis in adults with cold agglutinin disease.7 Phase 1 trials of this agent have been conducted in patients with chronic immune thrombocytopenia (NCT03275454) and with 1 of 3 other complement-mediated diseases (bullous pemphigoid, warm autoimmune hemolytic anemia, or ESRD) (NCT02502903).74,75
Avacopan is an oral C5a receptor antagonist that blocks the neutrophil activation and migration mediated by C5a.8 Avacopan was approved in 2021 in combination with standard-of-care (SOC) therapy for adults with severe active ANCA–associated vasculitis.8 Phase 2 trials of avacopan have been conducted for patients with C3G (NCT03301467), hidradenitis suppurativa (acne inversa) (NCT03852472), and IgA nephropathy (NCT02464891).76-78
Limitations of Current Therapeutics
Despite demonstrated benefits in various complement-mediated diseases, use of inhibitors targeting the classical/lectin and terminal pathways is associated with limitations.4-6,79,80 Some patients with PNH remain anemic and still require transfusions despite treatment with a C5 or C3 inhibitor.72,80
An additional key limitation of these agents is the increased risk of infections resulting from blockage of the terminal complement pathway, which plays a key role in immune surveillance.2,4-6 The prescribing information for eculizumab, ravulizumab, and pegcetacoplan all carry boxed warnings concerning the potential for serious and potentially life-threatening meningococcal infections.4-6 The prescribing information for pegcetacoplan also specifically warns about the potential for serious infections caused by encapsulated bacteria (eg, Streptococcus pneumoniae, Neisseria meningitidis, Hemophilus influenzae type B).6 Patients should receive a meningococcal vaccine at least 2 weeks before initiating therapy with any of these agents; however, the risk of serious infection is not eliminated by vaccination.4-6
Due to the risk for serious infections, eculizumab, ravulizumab, and pegcetacoplan are available only via a REMS (Risk Evaluation and Mitigation Strategy) program, which ensures that prescribers counsel patients on associated risks and that prevention strategies are in place.4-6 Although they do not carry a boxed warning, the prescribing information for sutimlimab and avacopan include warnings about the potential for serious infections.7-8 Use of sutimlimab may increase susceptibility to serious infections caused by encapsulated bacteria; pneumonia and urinary tract infections were the most common serious infections reported in patients treated with avacopan.7-8
Emerging Therapeutics Targeting the Alternative Pathway
Emerging agents targeting the alternative pathway of the complement system employ novel means of treating both rare diseases (eg, PNH, complement-mediated renal disorders) and more common conditions (eg, AMD).9-20 Preclinical data suggest that some of these agents are highly selective for the alternative pathway, leaving the classical and lectin pathways intact, which could lower infection risk compared with C3 and terminal complement inhibitors.4-6,9,19,21
Iptacopan
Iptacopan (LNP023) is an oral small-molecule factor B inhibitor that was granted Breakthrough Therapy Designation (BTD) by the FDA for the treatment of PNH in 2020.9,81 At that time, iptacopan also was granted Rare Pediatric Disease Designation for C3G.81 Based on the results of in vivo and in vitro studies, iptacopan is considered to be a highly potent factor B inhibitor capable of blocking the activation of the alternative pathway at the level of the C3 convertase.9 In addition, this agent is highly selective for factor B without having any inhibitory effects on factor D or blocking activation of the classical or lectin complement pathways.9 In the United States, iptacopan is being studied in patients with PNH, C3G, AMD, aHUS, primary IgA nephropathy, lupus nephritis, and idiopathic membranous nephropathy.10-16
The BTD of iptacopan for PNH was based on positive interim results from two phase 2 studies that evaluated the safety, efficacy, pharmacokinetics, and pharmacodynamics of iptacopan in 2 patient populations. The first trial (NCT03896152) assessed outcomes in patients with PNH and active hemolysis who were complement treatment-naïve, whereas the second trial (NCT03439839) studied iptacopan in patients with PNH and active hemolysis despite treatment with SOC anticomplement agents.81-83
NCT03896152 was a 2-cohort, open-label, proof-of-concept study across centers in Korea, Taiwan, Malaysia, and Singapore, in patients with PNH and active hemolysis and no prior complement inhibitor treatment within 3 months of enrollment.82 Based on data from 12 evaluable patients, all participants experienced reductions in serum lactate dehydrogenase (LDH) levels of at least 60% by week 12 compared with baseline, meeting the primary end point of the trial.82 This improvement corresponded with hematologic benefit including clinically meaningful improvement in mean Hb levels, and all but 1 patient (with preexisting myelodysplastic syndrome)remained transfusion-free until at least week 12.82 None of the patients experienced serious or severe infectious events.82
In NCT03439839, an open-label, single-arm, phase 2 proof-of-concept trial in Europe, iptacopan was evaluated as an add-on treatment to SOC anti-C5 treatment with eculizumab in 10 patients with PNH and signs of active hemolysis despite use of eculizumab.83 After 13 weeks of treatment with iptacopan, all patients experienced improvements in LDH levels (a marker of active hemolysis), with reductions of 34% to 81% compared with baseline levels.83 Additionally, significant improvements from baseline in Hb levels were observed in all patients in the absence of RBC transfusions (P < .0001).83 Data from follow-up demonstrated that 7 patients were able to discontinue eculizumab and continue iptacopan monotherapy while maintaining a hematologic response for at least 3 months.83 All patients were vaccinated against N meningitidis, H influenzae, and S pneumoniae infection, and no serious infectious events were reported.83
Results from the phase 3 APPLY-PNH trial (NCT04558918) were recently presented at the 64th ASH Annual Meeting and Exposition on December 13, 2022, in New Orleans, Louisiana.84 Patients (N = 97) were randomly assigned 8:5 to either receive iptacopan monotherapy (200 mg twice daily) or to continue SOC regimen of either eculizumab or ravulizumab for 24 weeks. More than half of the patients (64.9%) had previously received treatment with eculizumab and 35.1% had received treatment with ravulizumab (mean treatment duration: 4 years). Iptacopan monotherapy achieved both primary end points and showed superiority vs SOC. A total of 85% of the patients treated with iptacopan (51/60) experienced an Hb increase from baseline of at least 2 g/dL and 70% (42/60) achieved an Hb of at least 12 g/dL, irrespective of RBC transfusions, compared with none of the patients on SOC (0/35) (P < .0001 for both). No deaths or treatment discontinuations occurred. Patients treated with iptacopan more frequently reported headache (16.1% vs 2.9%) or diarrhea (14.5% vs 5.7%) and less frequently experienced infestations/infections (38.7% vs 48.6%) or clinical breakthrough hemolysis (3.5% vs 17.1%) than did those treated with SOC.84 The ongoing phase 3 APPOINT-PNH trial (NCT04820530) is examining the safety and efficacy of iptacopan 200 mg twice daily as a monotherapy in patients who are naïve to complement inhibitor therapy.85
Iptacopan also was investigated in an open-label, 2-cohort phase 2 study of patients with C3G (NCT03832114). Results presented at the American Society of Nephrology 2021 Annual Meeting showed that primary end points were met in both cohorts.86,87 Patients with C3G who had received kidney transplant (native C3G; n = 16) experienced a 45% reduction in proteinuria (measured by 24-hour urinary protein-to-creatine ratio) from baseline (P = .0003). Patients with C3G who had previously received kidney transplant, and for whom data were available (n = 7), experienced a significant reduction in C3 protein deposits (obtained from kidney biopsy at week 12 compared with baseline) compared with baseline (P = .0313).87 The phase 3 APPEAR-C3G trial (NCT04817618), currently in the recruiting stage, will evaluate the efficacy and safety of iptacopan compared with placebo and SOC therapy in adults with native C3G.11,88
Iptacopan therapy also demonstrated positive results in patients with IgA nephropathy.89 A phase 2 trial (NCT03373461) met its primary end point by demonstrating a significant dose-response reduction of proteinuria in patients treated with iptacopan (n = 87) vs placebo (n = 25) in patients with IgA nephropathy after 90 days of treatment (P = .038).89,90 The phase 3 APPLAUSE-IgAN (NCT04578834) trial is currently recruiting patients; investigators will evaluate the safety and efficacy of iptacopan in patients with primary IgA nephropathy receiving background angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy.15
IONIS-FB-LRX
IONIS-FB-LRX, an antisense oligonucleotide targeting complement factor B production by hepatocytes, is being studied for the treatment of geographic atrophy secondary to AMD and of primary IgA nephropathy.91,92 The results of a phase 1 trial (NCT03101878), found that multiple-dose subcutaneous administration of IONIS-FB-LRX was associated with dose-dependent reductions of factor B plasma levels of up to 72% in healthy individuals (N = 54).91 These results led to current recruiting for the adaptive design, double-masked, placebo-controlled, phase 2 GOLDEN study of IONIS-FB-LRX in patients with geography atrophy secondary to AMD.91,93 In NCT04014335, a phase 2 trial, among patients with primary IgA nephropathy, treatment with IONIS-FB-LRX was associated with positive changes in 24-hour urinary protein level at 29 weeks compared with baseline, meeting the trial’s primary end point.92,94 Based on these findings, plans for a phase 3 trial evaluating IONIS-FB-LRX in patients with IgA nephropathy are underway.92
Lampalizumab
Lampalizumab is an antigen-binding fragment that targets factor D, blocking activation of the alternative pathway at the level of C3 convertase.45,95 In the MAHALO phase 2 trial (NCT01229215), patients with geographic atrophy due to AMD (N = 129) were randomly assigned to receive intravitreal lampalizumab or sham injections.95 After 18 months, patients given lampalizumab monthly experienced a 20% reduction in the mean change in progression of geographic atrophy area compared with patients in the pooled sham group (80% CI, 4%-37%; P = .117), meeting the prespecified significance level for this proof-of-concept study.95
Lampalizumab was subsequently evaluated in Chroma (NCT02247479) and Spectri (NCT02247531), 2 identically designed, phase 3, randomized, multicenter trials in adult patients (aged ≥ 50 years) with bilateral geographic atrophy secondary to AMD and no prior or current evidence of choroidal neovascularization.96 A total of 906 patients in Chroma and 975 patients in Spectri were randomly assigned to different cohorts to receive lampalizumab or sham injections.96 The primary end point of adjusted mean change in geographic atrophy lesion area from baseline to 48 weeks was 1.93 to 2.09 mm2 across all groups in the 2 studies.96 Robustness assessments for this end point failed to demonstrate a benefit of lampalizumab over sham therapy.96 In clinical subgroup analyses, no consistent benefit in any of the subgroups was demonstrated for lampalizumab over sham.96 Because these two phase 3 trials failed to meet their primary end point, FDA approval for the use of lampalizumab in this setting is no longer being pursued.97
Danicopan
Danicopan (ALXN2040) is an oral, small-molecule, factor D inhibitor being studied in patients with PNH and in those with geographic atrophy due to AMD.18,19,98,99 In 2019, based on positive phase 2 (NCT03472885) trial data, danicopan was granted BTD by the FDA for the treatment of PNH as an add-on therapy to a C5 inhibitor.100,101 Danicopan was previously evaluated in patients with C3G, but phase 2 data demonstrated suboptimal clinical response with incomplete inhibition of the alternative pathway; thus, this agent is no longer being studied for this indication.102 Phase 1 safety, pharmacokinetic, and pharmacodynamic studies of danicopan have been completed in patients with hepatic or renal impairment (NCT03555539 and NCT04935294), although results are not currently available.103,104 A phase 2 trial evaluating danicopan in geographic atrophy due to AMD (NCT05019521) currently is recruiting participants.98
An international, single-arm, dose-finding, phase 2 trial (NCT03053102) evaluated danicopan in 10 patients with hemolytic PNH who were not receiving treatment with a complement inhibitor.105 The trial met its primary efficacy end point, with all patients demonstrating a significant reduction in mean LDH level from baseline to day 28 (P < .001).105 Danicopan was associated with an improvement in anemia, with mean Hb values increasing from 9.8 g/dL at baseline to 10.9 g/dL at day 28 and 11.5 g/dL at day 84 (all P < .005).105 Benefits also were observed in patient-reported outcomes, with the mean Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue score among patients improving by 9 and 13 points at day 28 and 84, respectively (P < .05).105 No severe infectious events were reported in this trial.105
A different multicenter, open-label, phase 2 trial (NCT03472885) evaluated danicopan as add-on therapy in 12 patients with PNH who had an inadequate response to eculizumab.18 Findings after 24 weeks of danicopan treatment demonstrated a significant increase in mean Hb value from baseline, with a mean increase of 2.4 g/dL (P = .0001).18 This improvement was observed by the second week in most patients and was sustained for the duration of the study.18 Although no change in the mean LDH level was noted, favorable results in other laboratory markers (eg, mean absolute reticulocyte counts, total bilirubin, direct bilirubin) were observed.18 Additionally, a clinically meaningful decrease in the number of patients requiring RBC transfusion was noted during the 24 weeks of treatment.18 Patient-reported outcomes also improved from baseline in patients treated with danipocan and included in the efficacy analysis (n = 11), with improvements in FACIT–Fatigue score noted at week 24 (P = .0191).18 One serious adverse event (ie, pneumonia from viral bronchitis) was reported in a patient with history of neutropenia, although it was deemed to be unlikely related to danicopan treatment.18
The ongoing, phase 3 ALPHA trial (NCT04469465) is evaluating danicopan vs placebo as add-on treatment to a C5 inhibitor (eculizumab or ravulizumab) in patients with PNH with clinically evident extravascular hemolysis (Hb ≤ 9.5 g/dL and absolute reticulocyte count ≥ 120 x 109/L).99 Based on a prespecified interim analysis, add-on danicopan was associated with results superior to those of placebo, with the trial meeting its primary end point of improved Hb levels from baseline to week 12.17 Additionally, add-on danicopan was associated with improvements over placebo in key secondary end points, such as avoidance of transfusion and change in the FACIT–Fatigue score from baseline.17
Vemircopan
Vemircopan (ALXN2050) is an oral factor D inhibitor that inhibits the alternative pathway in a near complete and sustained manner, as demonstrated in two phase 1 studies of healthy volunteers (NCT05047458 and NCT05047484).102,106-109 This agent is currently being studied in patients with lupus nephritis, IgA nephropathy, PNH, and generalized myasthenia gravis.110-112 A randomized, multicenter, placebo-controlled, phase 2 trial (NCT05097989) currently in the recruiting stage will evaluate vemircopan as an add-on treatment to SOC background therapy in adults with either lupus nephritis or IgA nephropathy.110 A randomized, placebo-controlled, phase 2 trial evaluating vemircopan in patients with generalized myasthenia gravis (NCT05218096) also is currently recruiting.112 Outside the United States, this agent is being studied as monotherapy in an ongoing phase 2 trial of patients with PNH (NCT04170023); it includes those who are treatment-naïve, those who have had an inadequate response to eculizumab, and those who previously received danicopan.111 In an interim analysis, among patients who had completed 12 weeks of vemircopan 120 mg monotherapy twice daily (n = 9), mean (SD) Hg increased by 3.9 g/dL (1.11 g/dL); no serious or treatment-emergent adverse events (TEAE) of grade 3 or higher, discontinuations, or deaths were reported, and headache–the most common TEAE–occurred in 36.4% of patients.113
BCX9930
BCX9930 is an oral factor D inhibitor that has demonstrated complete suppression of the alternative pathway and blockage of both erythrocyte hemolysis and accumulation of C3 fragments on PNH erythrocytes in preclinical studies.114 In a phase 1/2 trial (NCT04330534), BCX9930 treatment was associated with greater than 99% suppression of the alternative pathway among both healthy individuals and patients with C3G.115 The ongoing phase 2 REDEEM-1 and REDEEM-2 trials will evaluate BCX9930 monotherapy among patients with PNH and residual anemia despite treatment with a C5 inhibitor and those not receiving complement inhibitor therapy, respectively (NCT05116774 and NCT05116787).116,117 Outside the United States, BCX9930 is being evaluated in an ongoing, open-label, proof-of-concept, phase 2 trial (RENEW; NCT05162066) among patients with C3G, IgA nephropathy, or membranous nephropathy.118
GT005
GT005 is an investigational, 1-time gene therapy that works by increasing production of the factor I protein, leading to decreased levels of key downstream proteins associated with complement system activation.20 The ongoing phase 1/2 FOCUS trial (NCT03846193) is evaluating the safety, dose response, and efficacy of a single subretinal injection of GT005 administered to genetically defined patients with macular atrophy due to AMD.119 Interim data from 13 patients demonstrated that most patients (n = 11) experienced significant increases in levels of factor I compared with baseline (P = .002). Significant average decreases of 46% were observed for vitreous levels of Ba protein in 11 patients and C3 breakdown proteins in all 13 patients, compared with baseline (both P = .001).20 Investigators on the phase 2 HORIZON (NCT04566445) and EXPLORE (NCT04437368) trials are currently recruiting; each will evaluate 2 doses of a single subretinal injection of GT005 in patients with geographic atrophy secondary to AMD.120,121
CLG561
CLG561, an antiproperdin antigen-binding fragment that inhibits activation of the alternative pathway, was studied in patients with geographic atrophy.122,123 A phase 2, proof-of-concept study (NCT02515942) evaluated intravitreal injection of CLG561 as monotherapy and with LFG316, vs sham injections, in 114 patients with geographic atrophy.123 After 12 injections administered every 28 days, treatment with CLG561 failed to show efficacy for the primary outcome of change in geographic atrophy lesion size from baseline.123
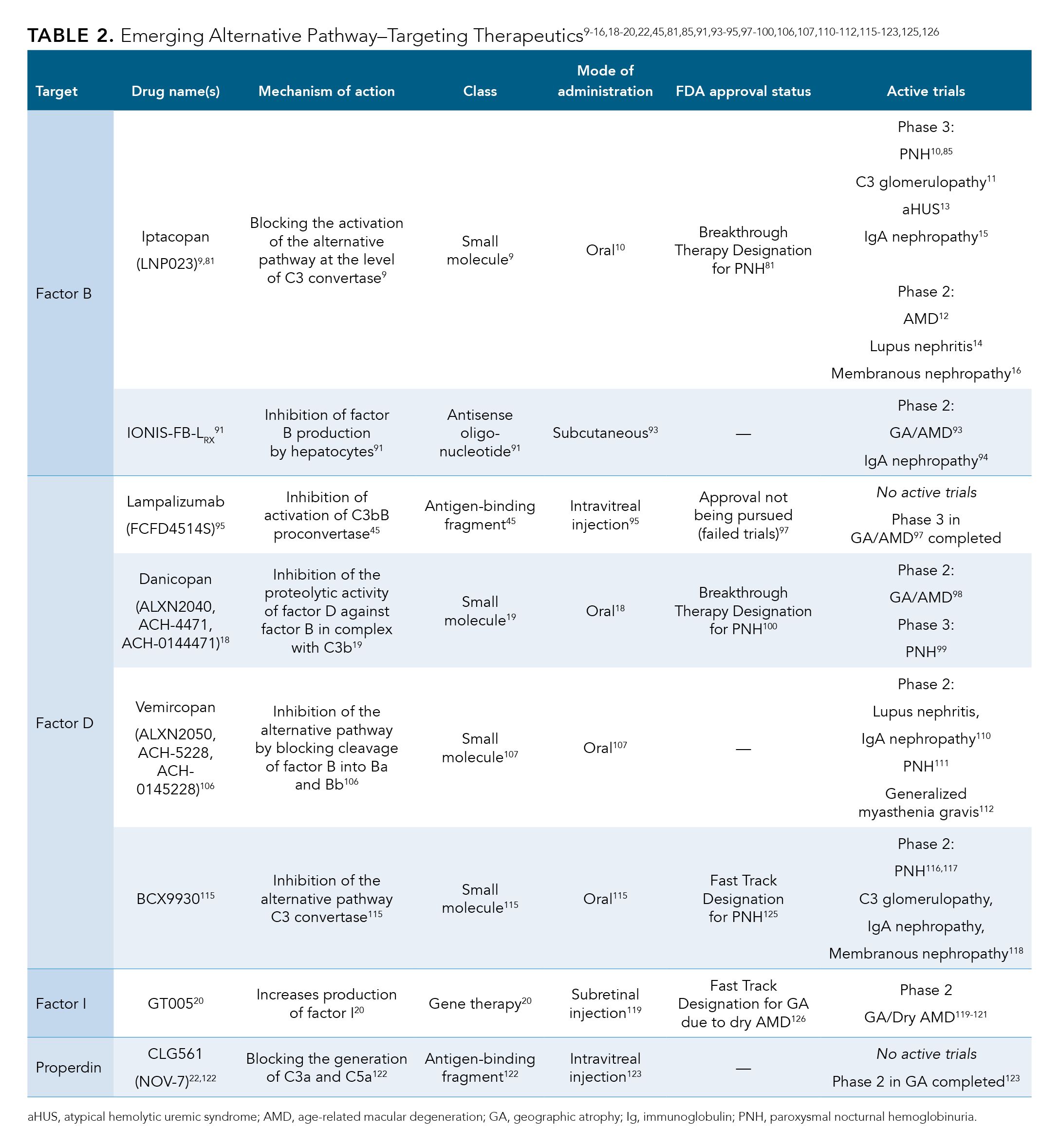
These alternative pathway–targeting therapeutics under development are included in Table 2.9-16,18-20,22,45,81,85,91,93-95,97-100,106,107,110-112,115-123,125,126
Conclusions
Complement system dysregulation is associated with a wide range of diseases.1-3 Currently available complement inhibitors have demonstrated treatment benefits in various diseases; still, their use is associated with limitations, including lack of response in certain patients and an increased risk of infections.4-6,72,79,80,124 Alternative pathway–targeting therapeutics are a promising novel strategy to treat many complement-mediated diseases.9-20 Some of these agents are highly selective for the alternative pathway; by leaving the classical and lectin pathways intact, their use could result in a lower infection risk compared with other complement inhibitors.4-6,9,19,21 Several ongoing trials are exploring the role of these promising agents in various diseases.10-16,98,99,120,121
References
1. Cortes C, Desler C, Mazzoli A, Chen JY, Ferreira VP. The role of properdin and Factor H in disease. Adv Immunol. 2022;153:1-90. doi:10.1016/bs.ai.2021.12.001
2. Ricklin D, Mastellos DC, Reis ES, Lambris JD. The renaissance of complement therapeutics. Nat Rev Nephrol. 2018;14(1):26-47. doi:10.1038/nrneph.2017.156
3. Pickering MC, D'Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84(6):1079-1089. doi:10.1038/ki.2013.377
4. Soliris. Prescribing information. Alexion Pharmaceuticals; 2020. Accessed December 22, 2022. https://alexion.com/Documents/Soliris_USPI.pdf
5. Ultomiris. Prescribing information. Alexion Pharmaceuticals; 2022. Accessed December 22, 2022. https://alexion.com/Documents/Ultomiris_USPI.pdf
6. Empaveli. Prescribing information. Apellis Pharmaceuticals; 2021. Accessed December 22, 2022. https://pi.apellis.com/files/PI_Empaveli.pdf
7. Enjaymo. Prescribing information. Bioverativ USA Inc; 2022. Accessed December 22, 2022. https://products.sanofi.us/enjaymo/enjaymo.pdf
8. Tavneos. Prescribing information. ChemoCentryx; 2021. Accessed December 22, 2022. https://www.chemocentryx.com/wp-content/uploads/2021/10/FINAL-PI-and-Med-Guide-7-Oct-21.pdf
9. Schubart A, Anderson K, Mainolfi N, et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc Natl Acad Sci U S A. 2019;116(16):7926-7931. doi:10.1073/pnas.1820892116
10. Study of efficacy and safety of twice daily oral LNP023 in adult PNH patients with residual anemia despite anti-C5 antibody treatment (APPLY-PNH). ClinicalTrials.gov. Updated November 25, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04558918
11. Study of efficacy and safety of iptacopan in patients with C3 glomerulopathy. (APPEAR-C3G). ClinicalTrials.gov. Updated November 25, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04817618
12. A masked, placebo-controlled study to assess iptacopan in age-related macular degeneration. ClinicalTrials.gov. Updated September 22, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT05230537
13. Efficacy and safety of iptacopan (LNP023) in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor therapy (APPELHUS). ClinicalTrials.gov. Updated December 21, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04889430
14. Study of efficacy and safety of LNP023 in participants with active lupus nephritis class III-IV, +/- V. ClinicalTrials.gov. Updated December 21, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT05268289
15. Study of efficacy and safety of LNP023 in primary IgA nephropathy patients (APPLAUSE-IgAN). ClinicalTrials.gov. Updated December 16, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04578834
16. Efficacy and safety of LNP023 compared with rituximab in subjects with idiopathic membranous nephropathy. ClinicalTrials.gov. Updated November 21, 2022. Accessed December 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04154787
17. Danicopan (ALXN2040) add-on to Ultomiris (ravulizumab-cwvz) or Soliris (eculizumab) met primary endpoint in ALPHA Phase III trial for patients with paroxysmal nocturnal hemoglobinuria who experience clinically significant extravascular hemolysis. News release. AstraZeneca. September 16, 2022. Accessed December 22, 2022. https://www.astrazeneca-us.com/media/press-releases/2022/danicopan-add-on-to-ultomiris-or-soliris-met-primary-endpoint-in-alpha-phase-III-trial-for-patients-with-paroxysmal-nocturnal-hemoglobinuria-who-experience-clinically-significant-extravascular-hemolysis.html
18. Kulasekararaj AG, Risitano AM, Maciejewski JP, et al. Phase 2 study of danicopan in patients with paroxysmal nocturnal hemoglobinuria with an inadequate response to eculizumab. Blood. 2021;138(20):1928-1938. doi:10.1182/blood.2021011388
19. Yuan X, Gavriilaki E, Thanassi JA, et al. Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica. 2017;102(3):466-475. doi:10.3324/haematol.2016.153312
20. Gyroscope Therapeutics announces presentation of positive interim phase I/II data for investigational gene therapy GT005 at Retina Society Annual Scientific Meeting. News release. Gyroscope Therapeutics. September 30, 2021. Accessed December 22, 2022. https://www.gyroscopetx.com/gyroscope-therapeutics-announces-presentation-of-positive-interim-phase-i-ii-data-for-investigational-gene-therapy-gt005-at-retina-society-annual-scientific-meeting/
21. Lewis LA, Panicker S, DeOliveira RB, Parry GC, Ram S. Effect of a C1s inhibitor on the efficacy of anti-capsular antibodies against Neisseria meningitidis and Streptococcus pneumoniae. Immunohorizons. 2019;3(11):519-530. doi:10.4049/immunohorizons.1900031
22. Freiwald T, Afzali B. Renal diseases and the role of complement: linking complement to immune effector pathways and therapeutics. Adv Immunol. 2021;152:1-81. doi:10.1016/bs.ai.2021.09.001
23. Loyet KM, Deforge LE, Katschke KJ Jr, et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(10):6628-6637. doi:10.1167/iovs.12-9587
24. Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804-2811. doi:10.1182/blood-2014-02-522128
25. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1-S276. doi:10.1016/j.kint.2021.05.021
26. Maillard N, Wyatt RJ, Julian BA, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26(7):1503-1512. doi:10.1681/ASN.2014101000
27. Bao L, Haas M, Quigg RJ. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol. 2011;22(2):285-295. doi:10.1681/ASN.2010060647
28. Grossman TR, Hettrick LA, Johnson RB, et al. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology. 2016;221(6):701-708. doi:10.1016/j.imbio.2015.08.001
29. Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52-64. doi:10.2353/ajpath.2007.060573
30. Batal I, Chalasani G, Wu C, Shapiro R, Bastacky S, Randhawa P. Deposition of complement product C4d in anti-glomerular basement membrane glomerulonephritis. Am J Kidney Dis. 2009;53(6):1098-1101. doi:10.1053/j.ajkd.2008.10.00
31. Thurman JM, Tchepeleva SN, Haas M, et al. Complement alternative pathway activation in the autologous phase of nephrotoxic serum nephritis. Am J Physiol Renal Physiol. 2012;302(12):F1529-F1536. doi:10.1152/ajprenal.00422.2011
32. Banda NK, Thurman JM, Kraus D, et al. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol. 2006;177(3):1904-1912. doi:10.4049/jimmunol.177.3.1904
33. Takeda K, Thurman JM, Tomlinson S, et al. The critical role of complement alternative pathway regulator factor H in allergen-induced airway hyperresponsiveness and inflammation. J Immunol. 2012;188(2):661-667. doi:10.4049/jimmunol.1101813
34. Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148(4):638-645. doi:10.1111/j.1365-2141.2009.07995.x
35. Brodsky RA. Complement in hemolytic anemia. Blood. 2015;126(22):2459-2465. doi:10.1182/blood-2015-06-640995
36. Elvington A, Atkinson C, Zhu H, et al. The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J Immunol. 2012;189(9):4640-4647. doi:10.4049/jimmunol.1201904
37. Bostanci N, Bao K, Li X, et al. Gingival exudatome dynamics implicate inhibition of the alternative complement pathway in the protective action of the C3 inhibitor Cp40 in nonhuman primate periodontitis. J Proteome Res. 2018;17(9):3153-3175. doi:10.1021/acs.jproteome.8b00263
38. Silasi-Mansat R, Zhu H, Popescu NI, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116(6):1002-1010. doi:10.1182/blood-2010-02-269746
39. Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J Neurosci. 2004;24(29):6457-6465. doi:10.1523/JNEUROSCI.0901-04.2004
40. Fonseca MI, Ager RR, Chu SH, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J Immunol. 2009;183(2):1375-1383. doi:10.4049/jimmunol.0901005
41. Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med. 2014;211(9):1793-1805. doi:10.1084/jem.20131902
42. Goodship TH, Cook HT, Fakhouri F, et al; Conference Participants. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017;91(3):539-551. doi:10.1016/j.kint.2016.10.005
43. Frazer-Abel A, Sepiashvili L, Mbughuni MM, Willrich MA. Overview of laboratory testing and clinical presentations of complement deficiencies and dysregulation. Adv Clin Chem. 2016;77:1-75. doi:10.1016/bs.acc.2016.06.001
44. Barratt J, Weitz I. Complement factor D as a strategic target for regulating the alternative complement pathway. Front Immunol. 2021;12:712572. doi:10.3389/fimmu.2021.712572
45. Katschke KJ Jr, Wu P, Ganesan R, et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J Biol Chem. 2012;287(16):12886-12892. doi:10.1074/jbc.M112.345082
46. PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Age-Related Macular Degeneration PPP 2019. American Academy of Ophthalmology. October 2019. Accessed December 21, 2022. https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp
47. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572. doi:10.1001/archopht.122.4.564
48. Holekamp N, Wykoff CC, Schmitz-Valckenberg S, et al. Natural history of geographic atrophy secondary to age-related macular degeneration: results from the prospective Proxima A and B clinical trials. Ophthalmology. 2020;127(6):769-783. doi:10.1016/j.ophtha.2019.12.009
49. Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754-1760. doi:10.1001/archopht.124.12.1754
50. Sarda SP, Heyes A, Bektas M, et al. Humanistic and economic burden of geographic atrophy: a systematic literature review. Clin Ophthalmol. 2021;15:4629-4644. doi:10.2147/OPTH.S338253
51. Anastasopoulos E, Yu F, Coleman AL. Age-related macular degeneration is associated with an increased risk of hip fractures in the Medicare database. Am J Ophthalmol. 2006;142(6):1081-1083. doi:10.1016/j.ajo.2006.06.058
52. Paroxysmal nocturnal hemoglobinuria. National Organization for Rare Disorders. 2019. Accessed December 21, 2022. https://rarediseases.org/rare-diseases/paroxysmal-nocturnal-hemoglobinuria/
53. Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry. Ann Hematol. 2020;99(7):1505-1514. doi:10.1007/s00277-020-04052-z
54. Cheng WY, Sarda SP, Mody-Patel N, et al. Real-world healthcare resource utilization (HRU) and costs of patients with paroxysmal nocturnal hemoglobinuria (PNH) receiving eculizumab in a US population. Adv Ther. 2021;38(8):4461-4479. doi:10.1007/s12325-021-01825-4
55. Kwon CS, Daniele P, Forsythe A, Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin A nephropathy. J Health Econ Outcomes Res. 2021;8(2):36-45. doi:10.36469/001c.26129
56. Chronic kidney disease basics. Centers for Disease Control and Prevention. Updated February 28, 2022. Accessed December 21, 2022. https://www.cdc.gov/kidneydisease/basics.html
57. Lloyd IE, Gallan A, Huston HK, et al. C3 glomerulopathy in adults: a distinct patient subset showing frequent association with monoclonal gammopathy and poor renal outcome. Clin Kidney J. 2016;9(6):794-799. doi:10.1093/ckj/sfw090
58. Nazareth TA, Kariburyo F, Kirkemo A, et al. Patients with idiopathic membranous nephropathy: a real-world clinical and economic analysis of U.S. claims data. J Manag Care Spec Pharm. 2019;25(9):1011-1020. doi:10.18553/jmcp.2019.18456
59. Greenbaum LA, Licht C, Nikolaou V, et al. Functional assessment of fatigue and other patient-reported outcomes in patients enrolled in the global aHUS registry. Kidney Int Rep. 2020;5(8):1161-1171. doi:10.1016/j.ekir.2020.05.003
60. Levy AR, Chen P, Johnston K, Wang Y, Popoff E, Tomazos I. Quantifying the economic effects of ravulizumab versus eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Med Econ. 2022;25(1):249-259. doi:10.1080/13696998.2022.2027706
61. Clarke AE, Yazdany J, Kabadi SM, et al. The economic burden of systemic lupus erythematosus in commercially- and medicaid-insured populations in the United States. Semin Arthritis Rheum. 2020;50(4):759-768. doi:10.1016/j.semarthrit.2020.04.014
62. Dall'Era M, Kalunian K, Eaddy M, et al. Real-world treatment utilization and economic implications of lupus nephritis disease activity in the United States. J Manag Care Spec Pharm. 2022;1-10. doi:10.18553/jmcp.2022.21496
63. Myasthenia gravis. National Organization for Rare Disorders. 2021. Accessed December 21, 2022. https://rarediseases.org/rare-diseases/myasthenia-gravis/
64. Howard JF Jr. Clinical overview of MG. Myasthenia Gravis Foundation of America. June 2015. Accessed December 21, 2022. https://myasthenia.org/Professionals/Clinical-Overview-of-MG
65. Mahic M, Bozorg AM, DeCourcy JJ, et al. Physician-reported perspectives on myasthenia gravis in the United States: a real-world survey. Neurol Ther. 2022;11(4):1535-1551. doi:10.1007/s40120-022-00383-3
66. Phillips G, Abreu C, Goyal A, et al. Real-world healthcare resource utilization and cost burden assessment for adults with generalized myasthenia gravis in the United States. Front Neurol. 2022;12:809999. doi:10.3389/fneur.2021.809999
67. FDA approves Alexion's Soliris for PNH. News release. FDA News. March 19, 2007. Accessed December 21, 2022. https://www.fdanews.com/articles/91963-fda-approves-alexion-s-soliris-for-pnh
68. Devalaraja-Narashimha K, Huang C, Cao M, et al. Pharmacokinetics and pharmacodynamics of pozelimab alone or in combination with cemdisiran in non-human primates. PLoS One. 2022;17(6):e0269749. doi:10.1371/journal.pone.0269749
69. Figueiredo MF. Phase 3 trials will test self-injectable crovalimab. News release. aHUS News. July 21, 2021. Accessed December 21, 2022. https://ahusnews.com/news/phase-3-trials-self-injectable-crovalimab-ahus-patients/
70. Search for clinical trials involving eculizumab and Solaris—35 accessed. ClinicalTrials.gov. December 15, 2022. https://clinicaltrials.gov/ct2/results?term=eculizumab&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=
71. Search for clinical trials involving ravulizumab and ALXN 1210—27 accessed. ClinicalTrials.gov. December 15, 2022. https://clinicaltrials.gov/ct2/results?term=ravulizumab&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=
72. Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028-1037. doi:10.1056/NEJMoa2029073
73. Search for clinical trials involving pegcetacoplan—11 accessed. ClinicalTrials.gov. December 15, 2022. https://clinicaltrials.gov/ct2/results?term=Pegcetacoplan&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=
74. Safety, tolerability and activity of BIVV009 in healthy volunteers and patients with complement mediated disorders (BIVV009-01). ClinicalTrials.gov. Updated April 25, 2022. Accessed December 15, 2022. https://clinicaltrials.gov/ct2/show/NCT02502903
75. A study to assess safety, tolerability, pharmacokinetics and pharmacodynamics of multiple-dose BIVV009 in participants with chronic immune thrombocytopenia (ITP). ClinicalTrials.gov. Updated April 25, 2022. Accessed December 15, 2022. https://clinicaltrials.gov/ct2/show/NCT03275454
76. Controlled trial evaluating avacopan in C3 glomerulopathy (ACCOLADE). ClinicalTrials.gov. Updated October 14, 2022. Accessed December 15, 2022. https://clinicaltrials.gov/ct2/show/NCT03301467
77. Evaluation of safety and efficacy of avacopan in subjects with moderate to severe hidradenitis suppurativa (AURORA). ClinicalTrials.gov. Updated October 28, 2022. Accessed December 15, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03852472
78. Complement inhibition in aHUS dialysis patients (ACCESS). ClinicalTrials.gov. Updated November 14, 2017. Accessed December 25, 2022. https://clinicaltrials.gov/ct2/show/NCT02464891
79. Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68(1):84-93. doi:10.1053/j.ajkd.2015.12.034
80. Shammo J, Gajra A, Patel Y, et al. Low rate of clinically evident extravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria treated with a complement C5 inhibitor: results from a large, multicenter, US real-world study. J Blood Med. 2022;13:425-437. doi:10.2147/JBM.S361863
81. Novartis investigational oral therapy iptacopan (LNP023) receives FDA Breakthrough Therapy designation for PNH and Rare Pediatric Disease Designation for C3G. News release. Novartis. December 16, 2020. Accessed December 21, 2022. https://www.novartis.com/news/media-releases/novartis-investigational-oral-therapy-iptacopan-lnp023-receives-fda-breakthrough-therapy-designation-pnh-and-rare-pediatric-disease-designation-c3g
82. Jang JH, Wong L, Ko BS, et al. Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study. Blood Adv. 2022;6(15):4450-4460. doi:10.1182/bloodadvances.2022006960
83. Risitano AM, Röth A, Soret J, et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open-label, single-arm, phase 2, proof-of-concept trial. Lancet Haematol. 2021;8(5):e344-e354. doi:10.1016/S2352-3026(21)00028-4
84. Peffault de Latour R, Roeth A, Kulasekararaj A, et al. Oral monotherapy with iptacopan, a proximal complement inhibitor of factor B, has superior efficacy to intravenous terminal complement inhibition with standard of care eculizumab or ravulizumab and favorable safety in patients with paroxysmal nocturnal hemoglobinuria and residual anemia: results from the randomized, active-comparator-controlled, open-label, multicenter, phase III APPLY-PNH study. Abstract presented at: 64th annual American Society of Hematology meeting; December 13, 2022; New Orleans, LA. Accessed December 19, 2022. https://ash.confex.com/ash/2022/webprogram/Paper171469.html
85. Study of efficacy and safety of twice daily oral iptacopan (LNP023) in adult PNH patients who are naive to complement inhibitor therapy (APPOINT-PNH). ClinicalTrials.gov. Updated November 25, 2022. Accessed December 19, 2022. https://clinicaltrials.gov/ct2/show/NCT04820530
86. Novartis iptacopan meets primary endpoints in phase II study in rare kidney disease C3 glomerulopathy (C3G). News release. Novartis. November 4, 2021. Accessed December 21, 2022. https://www.novartis.com/news/media-releases/novartis-iptacopan-meets-primary-endpoints-phase-ii-study-rare-kidney-disease-c3-glomerulopathy-c3g
87. Wong E, Nester C, Cavero Escribano T, et al. Iptacopan, a novel oral complement alternative pathway Factor B inhibitor, significantly reduces urinary protein excretion and C3 deposit scores in native and transplanted kidneys in patients with C3 glomerulopathy. Presented at the American Society of Nephrology (ASN) 2021 Annual Meeting; November 4, 2021; Virtual. Abstract PO2536. Accessed December 21, 2022. https://www.asn-online.org/education/kidneyweek/2021/program-abstract.aspx?controlId=3638501
88. Bomback AS, Kavanagh D, Vivarelli M, et al. Alternative complement pathway inhibition with iptacopan for the treatment of C3 glomerulopathy-study design of the APPEAR-C3G Trial. Kidney Int Rep. 2022;7(10):2150-2159. doi:10.1016/j.ekir.2022.07.004
89. Novartis announces iptacopan met phase II study primary endpoint in rare kidney disease IgA nephropathy (IgAN). News release. Novartis. June 6, 2021. Accessed December 21, 2022. https://www.novartis.com/news/media-releases/novartis-announces-iptacopan-met-phase-ii-study-primary-endpoint-rare-kidney-disease-iga-nephropathy-igan
90. Study of safety and efficacy of LNP023 in patients with kidney disease caused by inflammation. ClinicalTrials.gov. Updated October 7, 2022. Accessed December 15, 2022. https://clinicaltrials.gov/ct2/show/NCT03373461
91. Jaffe GJ, Sahni J, Fauser S, Geary RS, Schneider E, McCaleb M. Development of IONIS-FB-LRx to treat geographic atrophy associated with AMD. Invest Ophthalmol Vis Sci. 2020;61:4305. Abstract presented at: ARVO Annual Meeting; June 2020. Accessed December 21, 2022. https://iovs.arvojournals.org/article.aspx?articleid=2768483
92. Ionis partner licenses rare kidney disease treatment and will advance into phase 3 clinical study. News release. PRNewswire. July 11, 2022. Accessed December 21, 2022. https://www.prnewswire.com/news-releases/ionis-partner-licenses-rare-kidney-disease-treatment-and-will-advance-into-phase-3-clinical-study-301583315.html
93. GOLDEN STUDY: a study to assess safety and efficacy of multiple doses of IONIS-FB-LRx in participants with geographic atrophy secondary to age-related macular degeneration (AMD). ClinicalTrials.gov. Updated December 21, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT03815825
94. A study to evaluate the effectiveness and safety of IONIS-FB-LRx, an antisense inhibitor of complement factor B, in adult participants with primary IgA nephropathy. ClinicalTrials.gov. Updated August 12, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT04014335
95. Yaspan BL, Williams DF, Holz FG, et al; MAHALO Study Investigators. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9(395):eaaf1443. doi:10.1126/scitranslmed.aaf1443
96. Holz FG, Sadda SR, Busbee B, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136(6):666-677. doi:10.1001/jamaophthalmol.2018.1544
97. Genentech statement on chroma, the second phase III study for lampalizumab. News release. Genentech. November 9, 2017. Accessed December 21, 2022. https://www.gene.com/media/statements/ps_110917
98. A study of danicopan in participants with geographic atrophy secondary to age-related macular degeneration. ClinicalTrials.gov. Updated December 19, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT05019521
99. Danicopan as add-on therapy to a C5 inhibitor in paroxysmal nocturnal hemoglobinuria (PNH) participants who have clinically evident extravascular hemolysis (EVH)(ALPHA). ClinicalTrials.gov. Updated October 3, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT04469465
100. Achillion receives Breakthrough Therapy Designation from FDA for danicopan for treatment of paroxysmal nocturnal hemoglobinuria. News release. Globe Newswire. September 25, 2019. Accessed December 21, 2022. https://www.globenewswire.com/news-release/2019/09/25/1920498/0/en/Achillion-Receives-Breakthrough-Therapy-Designation-from-FDA-for-Danicopan-for-Treatment-of-Paroxysmal-Nocturnal-Hemoglobinuria.html
101. Study of danipocan in participants with paroxysmal nocturnal hemoglobinuria with inadequate response to eculizumab (PNH). ClinicalTrials.gov. Updated August 17, 2022. Accessed December 15, 2022. https://clinicaltrials.gov/ct2/show/NCT03472885
102. Dearment A. Alexion drops kidney disease program for drug that was part of $930M Achillion buyout last year. News release. MedCityNews. July 30, 2020. Accessed December 21, 2022. https://medcitynews.com/2020/07/alexion-drops-kidney-disease-program-for-drug-that-was-part-of-930m-achillion-buyout-last-year/
103. Study of danicopan in participants with hepatic impairment. ClinicalTrials.gov. Updated August 20, 2021. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT03555539
104. Study of danicopan in participants with normal kidney function and participants with kidney dysfunction. ClinicalTrials.gov. Updated June 23, 2021. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT04935294
105. Risitano AM, Kulasekararaj AG, Lee JW, et al. Danicopan: an oral complement factor D inhibitor for paroxysmal nocturnal hemoglobinuria. Haematologica. 2021;106(12):3188-3197. doi:10.3324/haematol.2020.261826
106. Thesaurus: vemircopan (code C174393). National Cancer Institute. Accessed October 19, 2022. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C174393
107. Achillion’s ACH-5228 achieves positive results in phase 1 multiple ascending dose study in healthy volunteers. News release. Globe Newswire. July 22, 2019. Accessed December 21, 2022. https://www.globenewswire.com/news-release/2019/07/22/1885664/0/en/Achillion-s-ACH-5228-Achieves-Positive-Results-in-Phase-1-Multiple-Ascending-Dose-Study-in-Healthy-Volunteers.html
108. A study of single-dose ALXN2050 in healthy adults. ClinicalTrials.gov. Updated September 17, 2021. Accessed December 19, 2022. https://clinicaltrials.gov/ct2/show/NCT05047458
109. A study of multiple doses of ALXN2050 in healthy adults. ClinicalTrials.gov. Updated September 17, 2021. Accessed December 19, 2022. https://clinicaltrials.gov/ct2/show/NCT05047484
110. Study of ALXN2050 in proliferative lupus nephritis (LN) and immunoglobulin A nephropathy (IgAN). ClinicalTrials.gov. Updated December 2, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT05097989
111. Study of the oral factor D (FD) inhibitor ALXN2050 in PNH patients as monotherapy. ClinicalTrials.gov. Updated October 24, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT04170023
112. Study of ALXN2050 in adult participants with generalized myasthenia gravis. ClinicalTrials.gov. Updated December 16, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT05218096
113. Browett PJ, Kulasekararaj A, Notaro R, et al. Vemircopan (ALXN2050) monotherapy in paroxysmal nocturnal hemoglobinuria: interim data from a phase 2 open-label proof-of-concept study. Oral abstract presented at: 64th annual American Society of Hematology meeting; December 10, 2022; New Orleans, LA. Accessed December 12, 2022. https://ash.confex.com/ash/2022/webprogram/Paper169301.html
114. BioCryst’s oral factor D Inhibitor (BCX9930) shows high potency and specificity for alternative pathway of complement. News release. Globe Newswire. December 6, 2020. Accessed December 21, 2022. https://www.globenewswire.com/news-release/2020/12/06/2140171/0/en/BioCryst-s-Oral-Factor-D-Inhibitor-BCX9930-Shows-High-Potency-and-Specificity-for-Alternative-Pathway-of-Complement.html
115. BioCryst presents data demonstrating > 99 percent suppression of alternative pathway complement activity with BCX9930 in C3G patients. News release. Globe Newswire. August 26, 2022. Accessed December 21, 2022. https://www.globenewswire.com/en/news-release/2022/08/26/2505233/29446/en/BioCryst-Presents-Data-Demonstrating-99-Percent-Suppression-of-Alternative-Pathway-Complement-Activity-with-BCX9930-in-C3G-Patients.html
116. BCX9930 for treatment of PNH in subjects with inadequate response to C5 inhibitor therapy (REDEEM-1). ClinicalTrials.gov. Updated December 16, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT05116774
117. BCX9930 for the treatment of PNH in subjects not receiving other complement inhibitor therapy (REDEEM-2). ClinicalTrials.gov. Updated December 16, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT05116787
118. BCX9930 for the treatment of C3G, IgAN, and PMN (RENEW). ClinicalTrials.gov. Updated November 22, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT05162066
119. FOCUS: first in human study to evaluate the safety and efficacy of GT005 administered in subjects with dry AMD. ClinicalTrials.gov. Updated December 1, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT03846193
120. HORIZON: a phase II study to evaluate the safety and efficacy of two doses of GT005. ClinicalTrials.gov. Updated August 2, 2022. Accessed October 25, 2022. https://clinicaltrials.gov/ct2/show/NCT04566445?term=NCT04566445&draw=2&rank=1
121. EXPLORE: a phase II study to evaluate the safety and efficacy of two doses of GT005 (EXPLORE). ClinicalTrials.gov. Updated December 5, 2022. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT04437368
122. Johnson L, Splawski I, Baker L, et al. Generation and characterization of CLG561: a fully-human, anti-properdin Fab for the treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(12):1117. Abstract presented at: ARVO Annual Meeting; May 1-5, 2016. Seattle, Washington. Accessed December 21, 2022. https://iovs.arvojournals.org/article.aspx?articleid=2559813
123. CLG561 proof-of-concept study as a monotherapy and in combination with LFG316 in subjects with geographic atrophy (GA). ClinicalTrials.gov. Updated May 30, 2019. Accessed December 21, 2022. https://clinicaltrials.gov/ct2/show/NCT02515942
124. Rondeau E, Scully M, Ariceta G, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97(6):1287-1296. doi:10.1016/j.kint.2020.01.035
125. FDA grants Fast Track Designation for BCX9930 in PNH. News release. Globe Newswire. August 31, 2020. Accessed December 21, 2022. https://www.globenewswire.com/news-release/2020/08/03/2071591/0/en/FDA-Grants-Fast-Track-Designation-for-BCX9930-in-PNH.html
126. Gyroscope Therapeutics granted FDA Fast Track designation for GT005, an investigational gene therapy for dry age-related macular degeneration. News release. Gyroscope Therapeutics. September 22, 2020. Accessed December 21, 2022. https://www.gyroscopetx.com/gyroscope-therapeutics-granted-fda-fast-track-designation-for-gt005-an-investigational-gene-therapy-for-dry-age-related-macular-degeneration/
