- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
The Economic and Societal Burden of Alzheimer Disease: Managed Care Considerations
To claim CE credit for this activity, please visit https://www.pharmacytimes.org/courses/a-supplement-to-the-american-journal-of-managed-care-advancing-alzheimer-disease-treatment-updates-and-insights-for-managed-care
ABSTRACT
Alzheimer disease (AD) is the most common cause of dementia and the sixth leading cause of death in the United States. Today, more than 6 million Americans are living with AD and that number is expected to increase to 13.8 million by 2060. The progressive debilitating nature of this illness and the absence of disease-modifying treatments contributes to a substantial economic and societal burden on the healthcare system. In 2022, the estimated healthcare costs associated with AD treatment were $321 billion, with costs projected to exceed $1 trillion by 2050. These cost-of-care projections are based on direct healthcare costs and are likely underestimated because indirect costs associated with AD treatment are usually not included. Indirect costs such as loss in productivity, diminished quality of life, and an increasing dependence on informal unpaid care provided by family caregivers augments the economic and societal burden of this disease. As drug development continues to evolve, the emergence of disease-modifying therapy may help to offset the burden associated with AD-related dementia. Managed care organizations are uniquely positioned to mitigate costs and positively impact outcomes through the promotion of disease awareness, early diagnosis, and treatment and disease management programs focused on multidisciplinary care coordination and caregiver support.
Am J Manag Care. 2022;28(suppl 10):S188-S196. https://doi.org/10.37765/ajmc.2022.89236
Introduction
Alzheimer disease (AD), a progressive, neurodegenerative brain disease leading to cognitive decline and dementia, is the sixth-leading cause of death among Americans.1 The risk of developing AD increases with age and disproportionately affects women, Black/African American individuals, and Hispanic individuals. Of the estimated 6.5 million Americans 65 years and older living with AD, 73% are 75 years or older. As the population continues to age, the number of individuals afflicted with AD is expected to more than double by the year 2060 to 13.8 million individuals.1
The life expectancy of an individual diagnosed with AD averages 4 to 8 years, with some living for 20 years after initial diagnosis.1 The long duration of illness before death contributes significantly to the economic and societal impact of AD, as much of that time is spent in a state of severe disability and dependence on caregivers.1,2
As the disease progresses, individuals with AD require increasing levels of medical care, caregiver support, and eventually long-term care that may include home health, assisted living, nursing care, and hospice. It is estimated that approximately 75% of people with AD will be living in a nursing home at age 80 compared with 4% of the general population by this age.1 The healthcare burden associated with AD is substantial in the latter years of the diagnosis and often requires a coordinated and multidisciplinary approach that spans multiple segments of the healthcare delivery spectrum, including patients, caregivers, medical professionals, and the nursing care system. While the direct costs of care are generally accounted for as actual medical care costs paid by the patients’ health insurance provider, the nondirect medical costs, out-of-pocket costs, and the indirect costs of caregiving are often borne by a spouse or family member and are usually unaccounted for in the total cost of care. Impact of caregiving on caregiver health is not widely studied and may be underestimated when determining the total cost of care in AD.1,2
Although current treatments do not delay the progression of the disease, early detection and treatment with current AD therapies may assist in improving patient care and quality of life (QOL) as well as economic and caregiver outcomes.3-6 New and emerging therapeutics that delay disease progression represent a critical unmet need for patients diagnosed with AD and may prove cost-effective by slowing the rate of cognitive decline and the associated healthcare costs.7
Economic Burden
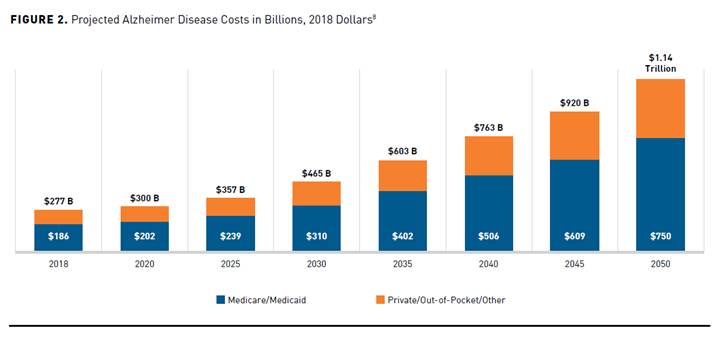
The progressive nature of AD and the lack of disease-modifying therapies contributes to the substantial economic and societal burden of illness on the US healthcare system. The total cost of treating individuals with AD and associated dementia is projected to increase from $321 billion in 2022 to over $1 trillion by 2050 (Figures 11 and 28). Recent aggregate cost-of-care data by payer type show Medicare and Medicaid assuming approximately two-thirds of these costs. The remaining costs include out-of-pocket costs, which are typically borne by the patient and their families, and “other” payment sources, defined as private insurance, health maintenance or managed care organizations, and uncompensated care.1
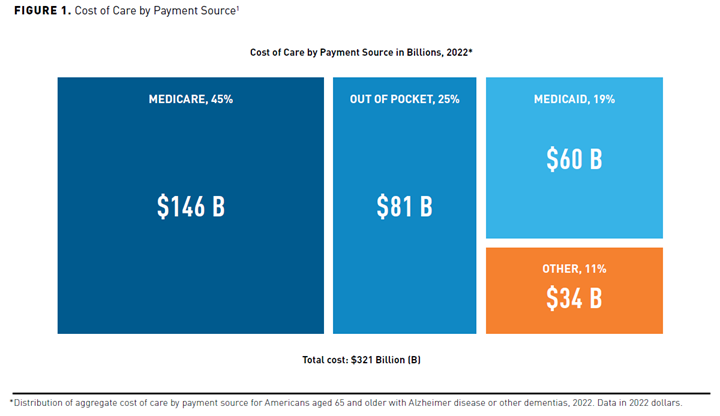
Cost of care is usually divided into direct and indirect costs. Direct medical costs associated with AD treatment include physician office visits, hospital admissions, emergency department visits, skilled nursing care, and medications.2,3 Long-term care including nursing homes and home healthcare account for the majority of direct costs associated with AD treatment. Direct nonmedical costs play an important role in AD as the disease progresses and patients require home health supervision, home safety modifications, full-time residential services, transportation, and supportive care. Indirect costs include costs associated with premature death, loss of productivity, and informal unpaid care costs or indirect costs that are predominantly borne by the patient’s family or caregiver.2,3
The average total annual costs in 2021 dollars for Medicare beneficiaries 65 years and older with AD or other dementias have been estimated to be $41,757, which is about 3 times higher than those without AD ($14,026). While the cost of care increased with an AD or dementia diagnosis, the percentage of Medicare care spend remained the same between the 2 groups. Medicare was responsible for 50% of that overall cost, while Medicaid costs accounted for a total of 23% for patients with AD or other dementias compared with 2% for patients without AD or other dementias. The average annual Medicaid costs for Medicare beneficiaries with AD were 22 times higher than those without AD ($6478 vs $291).1 A California-based Medicare fee-for-service study confirmed a similar finding with cost of AD or general dementia to be 2 to 3 times higher than patients without a dementia diagnosis.9
While Medicare costs may be higher for individuals with AD requiring medical care during the course of disease, studies indicate that during the last years of life preceding death, Medicare may not be the primary payer of the long-term care costs associated with treatment. Pyenson et al conducted a real-world, retrospective claims data analysis of 338,288 Medicare beneficiaries from 2006 to 2015 to evaluate the Medicare costs associated with a diagnosis of AD during the final years of life preceding death.10 The study found that an AD diagnosis only added about 11% per patient per year to Medicare costs over the final 8 years of life compared with non-dementia Medicare beneficiaries. After adjusting for age and risk, patients who died with a diagnosis of AD had costs (2015 US dollars) about $18,000 (10.9%) higher (P <.01) on average than decedents with no dementia over the last 8 years preceding death. This translated to an average annual cost differential of $2101 in 2015 dollars per Medicare beneficiary diagnosed with AD compared with those without a dementia diagnosis.10
The modest Medicare cost differential between patients with AD and those without AD can be explained by the coverage policy differences between Medicare and Medicaid. Medicare pays for medical care and only some short-term supportive care, it does not cover the cost of long-term care services including nursing home care. Medicaid pays for long-term care services and support including nursing home care when a beneficiary is sufficiently impoverished and meets Medicaid’s income and asset threshold.10,11
The associated Medicare costs for patients with AD in the years preceding death were generally related to the increased use of short-term supportive care such as skilled nursing facility and home health programs. As no disease-modifying medical treatments were available during the period of this claims analysis, the medical costs for Medicare were lower. The lower Medicare costs do not equate to low cost of care for the patients’ families or the public health system. Dementia caregivers bore nearly twice the average out-of-pocket costs of non-dementia caregivers ($12,388 vs $6667) and in 2022, the out-of-pocket cost for patients and their families has been estimated to comprise 25% of the total cost of care.1 In 2021, nursing home care averaged $95,000 to $108,405 per year and formal home care cost around $27 per hour, or roughly $56,160 annually for 40 hours in home care per week.12 Patients and their families may incur substantial out-of-pocket costs for long-term care services until the Medicare beneficiary qualifies for Medicaid.6,10 Patients with AD and other dementias were about 60% more likely to be dual eligible for Medicare and Medicaid versus patients without dementia and at the time of death, nearly twice as many with AD were dual eligible.10
The findings by Pyenson et al support previous research findings conducted by Kelley et al; in a retrospective sample of Health and Retirement Study Medicare claims spending over the last 5 years of life, Kelley et al showed the mean adjusted total healthcare spending in the last years of life was $287,038 in patients with dementia compared with $183,001 in other disease groups (eg, cancer, heart disease).13 However, the mean adjusted Medicare spending in the last 5 years was similar across groups: $86,430 (dementia) and $98,326 (non-dementia), while average Medicaid, out-of-pocket, and informal care (unpaid) costs were higher for the dementia group than for the non-dementia group.13
Another component of costs includes indirect costs that are incurred due to loss in productivity as well as cost of informal unpaid care provided by family and friends. The productivity loss is not limited to the patient who experiences disability or dies prematurely but also the caregiver who provides care to the ailing patient at their own expense. Current estimates of the lifetime costs of care may underestimate the impact caregiving has on the caregiver’s health and workplace productivity.2 The informal cost of care is usually measured by assigning a monetary value to the time lost due to unpaid caregiving. Based on the 2022 Alzheimer’s Association report, an estimated 11.3 million family and unpaid caregivers of individuals with AD or other dementias provided an estimated 16 billion hours of informal (unpaid) assistance valued at approximately $271.6 billion.1
The total lifetime cost of care for a patient with dementia is estimated at $412,936 in 2022 dollars, with 70% of those costs borne by the family caregivers in the form of unpaid caregiving and out-of-pocket expenses for items ranging from home health support to medications.1,14,15 Kelley et al found that the average cost of informal care for patients with dementia was more than double the corresponding cost of care for patients without dementia, $83,022 versus $38,272 in 2015 dollars corresponding to a total of $102,385 and $47,198 in 2022 dollars based on a 23.3% cumulative rate of inflation.1,13,15
In addition to staggering indirect costs, family caregivers are increasingly vulnerable to health consequences of the chronic stressors associated with AD caregiving.16 As individuals with AD continue to progress to more functional disability and cognitive impairment, the dependence on the caregiver grows. The current healthcare system relies heavily on informal unpaid caregivers, many of whom are themselves socioeconomically and medically vulnerable.17,18 Women represent 58% of all dementia caregivers and 42% of caregivers have a household income of $50,000 or less.17 Dementia caregivers provide more hours of care per week than their non-dementia counterparts (49.7 vs 37.7 hours) and were twice as likely to provide 40 hours of care per week.19 As a result, caregivers are more likely to experience disruptions to their work schedule, reduce hours worked, or leave the workforce.1,17
The high burden of care over a long period of time can take a significant mental and physical toll on dementia caregivers.1,17 Caregivers experience higher stress related to the unique aspects of dementia care and are at higher risk for cardiovascular (CV) disease, diabetes, obesity, cancer, and depression.1,19,20
A retrospective cohort study using Medicare Advantage Prescription Drug Plan members with a diagnosis of AD compared with those without a diagnosis found that household members caring for patients with AD had significantly higher average annual healthcare costs ($7168 ± $10,050 vs $6301 ± $8311; P <.01).16 As the population ages and families are less able to bear the cost of long-term care, the current system may be unable to meet the growing demand without alternative care programs and sustainable financing. Policymakers and managed care organizations seeking strategies to manage AD should consider healthcare strategies that allow patients to remain at home longer while providing support services to reduce caregiver burden.3,18
Importance of Early Diagnosis and Treatment
The goals of treatment for patients diagnosed with AD are to (1) maintain QOL, (2) maximize function in daily activities, (3) enhance cognition, mood, and behavior, (4) foster a safe environment, and (5) promote social engagement.21 Optimization of these goals is predicated on a timely and accurate diagnosis by a medical provider, engagement of patient and family support, and the initiation of safe and effective pharmaceutical and nonpharmaceutical interventions.
Current evidence indicates that only half of those with AD dementia have received a diagnosis.22 Access to providers trained in making a timely and accurate AD diagnosis is a public health challenge. The International Working Group (IWG) recommends that a confirmed diagnosis of AD must include both the presence of biomarkers (β-amyloid [Aβ] and tau positivity) and specific clinical phenotypes common to AD (amnestic AD, logopenic variant primary progressive aphasia, and posterior cortical atrophy). Diagnostic testing for the presence of Aβ plaques and tau protein tangles is confirmed in the cerebrospinal fluid or through a positron emission tomography (PET) scan.23 These testing methods are expensive, invasive, and have limited availability.24 Advanced usage of blood-based AD biomarker testing is an important development that would increase the availability of a more accessible, noninvasive, and inexpensive screening tool.25,26 Recently, a novel blood test was shown to accurately detect the presence of Aβ in the brain and demonstrated even greater accuracy in the presence of the APOE gene linked to AD.27 PET scans and cerebrospinal fluid biomarker testing are not currently covered by Medicare under most circumstances and the blood test is not currently FDA approved or accepted for coverage by Medicare.28,29
Common barriers to early diagnosis include lack of primary care provider training in dementia diagnostics and scoring, insufficient time during medical visits to conduct an assessment, reluctance of patients and caregivers discussing cognitive impairment during visits, inconsistent insurance coverage for confirmatory biomarker testing or imaging, and a shortage of medical specialists (neurology and geriatrics).3,22,30
Approximately 85% of patients are first diagnosed by a nonspecialist or primary care physician, whereas the remaining 15% receive their initial diagnosis from a specialist.1,31 One study examined the differential impact among 16,554 Medicare beneficiaries who received an initial AD diagnosis by a specialist versus a nonspecialist.31 The study researchers found the group initially diagnosed by a specialist (15.7%) were younger, more likely to be male, and exhibited higher rates of comorbidities but incurred lower costs in the year immediately following the initial diagnosis. The study further suggested that patients who consulted a specialist upon initial indication of cognitive decline led to a timelier diagnosis of AD and significantly lower all-cause medical costs in the first year after diagnosis.31
Early diagnosis of AD and the subsequent access to treatments, enrollment in clinical trials, and adjustment of modifiable risk factors may contribute to improved patient care and QOL. In the absence of a disease-modifying treatment or cure, reducing the risk of developing dementia takes on greater importance when considering treatment goals.32 The Alzheimer’s Association evaluated and found sufficient evidence to support the link between modifiable risk factors and the risk of cognitive decline. Specifically, they concluded that regular physical activity, management of CV risk factors (diabetes, obesity, smoking, and hypertension), a healthy diet, and mental/intellectual stimulation may reduce the risk of cognitive decline.32 As many as 90% of Medicare beneficiaries with AD or other dementia have at least 1 other chronic condition and are 2.7 times more likely to have 4 or more chronic conditions than Medicare beneficiaries without dementia.1 Therefore, a reduction in these modifiable risk factors may yield a reduction in the prevalence of dementia and an associated cost reduction as well.
Lin and colleagues developed a cohort-based simulation model using data from 1997-2005 Medicare Current Beneficiary Survey Cost and Use Files to examine the relationship between risk factors for dementia, the onset and duration of dementia, and its cost impact to Medicare and Medicaid.33 The model determined that a 10% reduction in the prevalence of CV disease decreased the risk of dementia by 0.6%, delayed the onset of dementia by 0.1 year, and reduced the time spent living with it by 0.03 years, which yielded a substantial lifetime savings for both Medicare ($1413 per capita) and Medicaid ($1208 per capita). Across the US population of an estimated 76 million baby boomers (a person born between 1946 and 1964), a 10% reduction in CV disease would correspond to a savings of approximately $20 billion for Medicare and $17 billion for Medicaid. The researchers also found that reductions in body mass index among overweight or obese individuals, diabetes, or hypertension also reduced the duration of dementia and delayed its onset by smaller but still significant amounts. A 10% reduction in body mass index was estimated to save Medicare $6 billion and Medicaid $35 billion over the lifetime of the baby boomer population because of lower dementia costs.33
A US Medicare fee-for-service claims database analysis among beneficiaries from January 2011 to June 2014 evaluated the relationship between treated and untreated patients with a new diagnosis of AD on patient care outcomes and cost.34 After controlling for comorbidities, the study researchers demonstrated that among the 8995 newly diagnosed patients with AD between 65 and 100 years of age, those that were treated saw improvements in survival and 20% reduction in institutionalization and incurred lower annual all-cause costs ($25,828 vs $30,110; P = .0162).34 Another claims analysis using data from MarketScan Commercial and Medicare databases from 2010 to 2016 found that patients who initiated treatment before or concurrent with an AD diagnosis experienced 9% to 19% lower healthcare costs in the year following diagnosis than those who did not receive treatment.35
Several economic models have been developed to determine the cost-effectiveness of early treatment with disease-modifying therapies on the overall cost burden associated with AD when one becomes available. Green et al developed a decision-analytic model using data from the US National Alzheimer’s Coordinating Center to investigate the cost-effectiveness of hypothetical treatment at an annual cost of $5000 per patient and found an increase in mean life expectancy, reduction in time spent with dementia, and increase in 5- and 10-year survival at a cost per quality-adjusted life-year gained for treatment of $50,542.36 A Monte Carlo cost-benefit analysis conducted by Weimer et al determined the benefit of early diagnosis, and the combination of a disease-delaying treatment and a caregiver intervention program led to a net positive social benefit and net fiscal cost savings.6 The results demonstrated the highest net benefit at the initial symptomatic stage for a 70-year-old married woman with a Mini-Mental State Exam score of 28 with a mean net social, state fiscal, and federal fiscal benefit of $125,000, $16,000, and $34,000, respectively.6 Currently, no FDA-approved treatment has conclusively demonstrated disease-modifying benefit; however, future disease-modifying treatments in development have the potential to be cost-effective in delaying progression of AD, reducing long-term care costs, and decreasing caregiver burden, if priced responsibly.6
Creating a Viable Patient Care Ecosystem
The patient journey in AD requires a coordinated approach to managing the health consequences of AD and the underlying comorbidities that impact the disease process. Even small improvements in modifiable risk factors have shown to have a positive effect on the underlying dementia process and have demonstrated significant cost savings to healthcare systems and payers.33
As patients with AD progress in their disease, they require increasing levels of care and support to conduct activities of daily living. People with AD require care from a wide variety of healthcare professionals, from nursing professionals, pharmacists, and home healthcare workers to physician specialists such as geriatricians and psychiatrists; however, care is often provided in a fragmented and uncoordinated fashion.20 Care coordination is best defined as “…the deliberate organization of patient care activities between 2 or more participants involved in a patient’s care to facilitate the appropriate delivery of healthcare services.”20 Care coordination has the potential to address multidisciplinary needs of individuals with AD and informal caregivers and improve health outcomes for both parties. The Veterans Affairs in combination with Alzheimer’s Association chapters found that a care coordination program effectively reduced the number of hospital and emergency department visits among the veterans based on caregiver reports.37 Care coordination may also reduce Medicare and Medicaid expenditures by ensuring timely care and services that keep patients healthier.38
Currently, the system relies heavily on informal, unpaid care for patients with AD; however, based on US Census data, which shows a slight increase in the aged 18 to 64 years population from approximately 202 million to 225 million by 2050, there will not be sufficient caregivers to care for the aging.39 Therefore, it will become extremely important to increase the number of trained direct healthcare workers to meet this growing demand. Recent projections show a nationwide increase of 34.5% in demand for home health and personal care aides to meet the needs of the aging population by 2028.1 The inevitable care gap that will occur over the next few decades may jeopardize the quality of care the patient will receive and may have a negative personal impact on patients and caregivers. As disease-modifying agents become available over the next few years, there may not be enough specialists to diagnose and treat patients or enough infusion centers across the county to provide treatment. An analysis conducted by Liu et al found that patients would have to wait an average of 18.6 months to receive treatment.40 As most primary care providers are not trained or comfortable in diagnosing AD and related dementia, the burden of care management and treatment would continue to fall on the specialists. Given the current shortage of specialists in the United States, the current number of geriatricians would have to increase by 900% to meet just 30% of the demand by 2050.1
The current constraints on the healthcare system driven in part by population growth and the imminent shortage of specialists and home health professionals demands the formation of a patient-centric integrated multidisciplinary care team. The IWG of diverse experts in various specialties, including neurology, geriatrics, nursing, pharmacology, and psychiatry, established a consensus recommendation to create a blueprint for a patient-centric diagnosis journey that supports awareness of early detection of AD and access to resources through the coordination of an integrated multidisciplinary dementia care team. The program emphasizes the need to educate future dementia-trained healthcare providers who can confidently stand in the gap and provide services to the aging population afflicted with AD-related dementia. Additionally, the IWG recommended an expansion of reimbursement codes to allow coverage of a diagnostic workup aimed at differentiating AD from other cognitive disorders, establishing a path for early-stage diagnosis of AD.41 Furthermore, a workgroup established by the Gerontological Society of America recommends that all Medicare beneficiaries with a diagnosis of AD or related dementia be referred to all appropriate and available community-based services to learn more about the disease process and how to navigate a future with a dementia diagnosis. These resources may include, but are not limited to, local area agencies specializing in aging and AD-related dementia, such as the Area Agency on Aging, local chapters of the Alzheimer’s Association, state aging and disability resource centers, as well as the American Association of Retired Persons, Alliance for Aging Research, and the Alzheimer’s Foundation of America.42 Pharmacists may be well positioned to support AD disease awareness and patient-centric education in the community as well as medication management and optimization to improve adherence and reduce medication adverse effects for aging patients.43,44
A timely and accurate diagnosis is critical to the development of an effective care plan, which requires coordination among patients, caregivers, members of the multidisciplinary healthcare team, and payers. An analysis froma pilot program conducted in 2020 confirmed that patients with AD and their caregivers who enrolled in a care coordination program were more likely to benefit from improved patient AD diagnosis and comorbidity documentation during medical visits.20 Additionally, the caregivers enrolled in this pilot program were found to have a reduction in emergency department use and inpatient care for depression (22% in the pre-intervention period vs 11% in the post-intervention period). The results were not statistically significant based on the small number of caregivers in the study arm (n = 63); however, the results are qualitatively important to encourage the exploration of more care coordination programs that may serve to improve the health of both patients diagnosed with AD and their caregivers.20
Improving Quality of Life for Patients With Alzheimer Disease
Potentially avoidable hospitalizations (PAHs) are admissions to a hospital for chronic or acute illnesses that would have otherwise been prevented with successful management in outpatient settings. PAHs are considered indicators of quality of care provided in ambulatory settings.45 In 2013, 1 in 10 patients with AD experienced 1 PAH accounting for nearly $2.6 billion in Medicare expenditures and as many as 26% of patients with AD experience a fall-related hospitalization.1,46 PAH-related falls were found to be 3 times higher among patients with AD in the year before their diagnosis.45 Implementation of pharmacist-driven medication therapy management programs may help to reduce medication-related adverse effects and prevent falls, improve adherence to therapy, and reduce the risk of complications from other comorbidities.43,44 Studies suggest comorbidity management remains suboptimal among many patients with AD, especially those with more advanced disease. Improving the symptoms of AD and related dementia may contribute to improvements in patient outcomes, QOL, and a reduction in overall healthcare costs.45,46
Optimal management of the consequences of dementia, particularly agitation, constitutes a major clinical challenge to avoid or delay institutionalization and improve QOL for patients. Early diagnosis may allow patients to receive treatments that may improve the symptoms of their disease but not alter the course of their disease process. For the treatment of cognitive symptoms, current pharmaceutical agents approved for use in patients in various stages of AD include cholinesterase inhibitors (donepezil, galantamine, rivastigmine) and memantine. These agents provide symptomatic relief by improving cognitive symptoms of memory loss and confusion, but do not stop disease progression. Promising disease-modifying agents that target the inflammatory processes, tau proteins, and Aβ proteins are currently under investigation.3,40,47
Nonpharmacologic therapy may be helpful in improving the QOL for some patients. Music therapy was found to be optimal for the management of agitation in institutionalized patients with moderately severe and severe AD, while therapeutic touch has been found to be effective for reducing the physical nonaggressive behaviors.48 Outdoor activities were found to be more efficacious than antipsychotics for managing physical aggression.49 Although AD and related dementia are known to negatively impact QOL, there is a paucity in data-quantifying costs associated with decreased QOL in the patient.3
The impact of COVID-19 on the QOL of patients with AD was detrimental based on several recently published findings. In 2020, COVID-19 was the third leading cause of death in the United States, pushing AD from sixth to seventh.1 COVID-19 increased the case fatality rate among patients diagnosed with AD compared with those without an AD diagnosis or with other forms of dementia not related to AD.50 These findings suggest that patients with AD were particularly vulnerable to the effects of the virus, SARS-CoV2, and its ability to invade the central nervous system. Individuals who were infected with COVID-19 frequently exhibited neurological impairments that may be further compounded in neurodegenerative conditions such as AD.50 An observational study performed by Matia-Guiu et al found living in care facilities to be a significant risk factor associated with death due to COVID-19 infection, especially among elderly patients with a diagnosis of AD.51 Patients residing in long-term care facilities experienced higher mortality rates compared with those who lived independently (40% vs 2.2%; P <.001).51
Managed Care Consideration for Alzheimer Disease
In 2018, the US National Institute on Aging and Alzheimer’s Association proposed a departure away from a clinical-biological diagnosis of AD to a biological definition based purely on the presence of biomarkers for research purposes.23 This change in definition of AD engendered substantial debate on the proper use of biomarkers to diagnose disease and use clinical symptoms and phenotypes for staging the disease. Three years later, the IWG convened to reevaluate the appropriate application of biomarkers to the diagnosis and recommended that the diagnosis of AD be a combination of specific clinical features based on commonly recognized phenotypes associated with AD and the positivity of both Aβ and tau biomarkers. The IWG concluded that the presence of biomarkers in cognitively impaired individuals alone was not predictive of AD diagnosis. Several studies indicate that the presence of biomarkers Aβ and tau is insufficient to predict the occurrence of symptoms (mild cognitive impairment or dementia) in individuals without evidence of clinical impairment. Individuals with the presence of biomarkers alone without the clinical features of AD should only be considered to be at risk for progression to AD.23 The appropriate application of biomarkers in AD prognosis becomes increasingly important with the emergence of new therapies shown to reduce biomarker levels. Treatments in development to date have been based on the amyloid cascade hypothesis and tau biology, with Aβ accumulation the main target of most drugs tested for AD over the past 20 years.
In June 2021, the FDA granted approval of aducanumab (Aduhelm), a human, immunoglobulin gamma 1 monoclonal antibody directed against aggregated soluble and insoluble forms of Aβ for the treatment of patients with AD using an accelerated approval process.52,53 This FDA approval was based on surrogate marker reduction in Aβ plaques on a PET scan rather than demonstration of clinical benefit in delaying progression of cognitive impairment or dementia.52,54
This FDA approval garnered public attention as well as scrutiny. The decision ran counter to the FDA’s advisory committee of experts who voted 10 of 11 against approval. The controversial approval prompted 3 committee members to resign from the agency over the lack of efficacy data supporting clinical benefit and drug safety concerns.55-57 Furthermore, public criticism was levied against the manufacturer who initially priced the monthly 10 mg/kg intravenous infusion at an annual cost of $56,000.7,58 After several months of slow market uptake of the drug, limited insurance coverage, and low sales, the company changed their pricing policy and reduced the cost by 50% for an annual price of $28,200.59 Economic analysis conducted by the Institute for Clinical and Economic Review (ICER) concluded that the evidence was insufficient to determine that the clinical benefit of aducanumab outweighs its risks or that it reduces progression of AD in patients with mild cognitive impairment and mild AD. The ICER further concluded that for aducanumab to be cost-effective, it would have to be price adjusted to an annual cost of $2950 to $8360, a reduction of 85% to 95% of the initial $56,000 price.7 Additional studies demonstrating clinical efficacy of patient outcomes and cost-effective analyses will be integral to determine aducanumab’s place in the management of AD.
The focus then shifted to the Centers for Medicare & Medicaid Services (CMS) for coverage guidance, as most of the eligible candidates for drug therapy with aducanumab would be Medicare beneficiaries. On April 7, 2022, CMS released its National Coverage Determination policy of aducanumab and any future FDA-approved monoclonal anti-amyloid antibodies to allow coverage with evidence development, essentially restricting coverage to patients enrolled in CMS-approved or National Institutes of Health-supported clinical trials.60 The decision from CMS to restrict use was based on the fact that existing clinical trials had not demonstrated unequivocally that the anti-amyloid monoclonal antibodies resulted in a clinically meaningful improvement in health outcomes for patients with AD and ensured patient benefits outweighed the safety risk.61,62
The CMS decision is likely to have far-reaching implications within the industry regarding access and coverage. Private payers often use CMS guidance when drafting coverage determinations and drug coverage policies. It is highly likely that national private insurers will restrict coverage based on insufficient evidence of efficacy and require patients to enroll in a clinical trial as a prerequisite to treatment.63 State-sponsored Medicaid programs may still be required to cover aducanumab, and thus would be financially responsible for the entire cost of aducanumab for dual Medicare- and Medicaid- eligible patients. However, state-sponsored Medicaid programs may also follow CMS guidance and consider restricting coverage based on insufficient evidence of clinical benefit, especially in the face of limited fiscal resources.
Manufacturers in process of developing monoclonal antibodies for AD treatment quickly reconsidered their decision to bring such products to market without proven clinical benefit.61,62 As manufacturers reconsider the timing of their new drug applications, it is important for managed care organizations and governmental payers to implement healthcare programs that would promote early diagnosis and treatment in addition to AD management programs to improve disease awareness, keep patients healthier, and reduce caregiver burden.
Conclusions
AD is a progressive, debilitating disease associated with substantial economic and public health burden. With the total direct cost of care estimated to rise to over $1 trillion over the next few decades and the expected population growth over the next several years, it is imperative that the US healthcare system prepare for the demand in healthcare workers and address the shortage of physician specialists. Pharmacists providing medication therapy management are well suited to step into the gap and help patients and caregivers better manage the disease process, identify medication-related issues, provide care coordination, and follow up when necessary. The emergence of disease-modifying therapies may help to preserve cognitive function in patients with AD by delaying disease progression, but whether these therapies will reduce the overall healthcare costs associated with AD remains to be seen. Determining the value of emerging novel therapeutics by payers will require clinical trial end points that demonstrate evidence of delaying disease progression reduction compared with merely a reduction in biomarkers.
Author affiliation: Anita Pothen Skaria, PharmD, is Director, Clinical Pharmacy Services, Centene Corporation, Boynton Beach, FL.
Funding source: This activity is supported by an educational grant from Lilly.
Author disclosure: Dr Skaria has the following relevant financial relationship with a commercial interest to disclose: Stock/Shareholder: Centene
Authorship information: Analysis and interpretation of data; concept and design; critical revision of the manuscript for important intellectual content; drafting of the manuscript; supervision.
Address correspondence to: Anita.Skaria@Centene.com
Medical writing and editorial support provided by: Shetal Desai, PharmD
REFERENCES
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.102/alz.12638
- Deb A, Thorton, JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing Alzheimer’s disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):189-202. doi:10.1080/14737167.2017.1313118
- Wong W. Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care. 2020;26(8 Suppl):S177-S183. doi:10.37765/ajmc.2020.88482
- Rasmussen J, Langerman H. Alzheimer’s disease-why we need early diagnosis. Degener Neurol Neuromuscl Dis. 2019;9:123-130. doi:10.2147/DNND.S228939
- Dubois B, Padovani A, Scheltens P, Rossi A, Dell’Agnello G. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49(3):617-631. doi:10.3233/JAD-150692
- Weimer DL, Sager MA. Early identification and treatment of Alzheimer’s disease: social and fiscal outcomes. Alzheimers Dement. 2009;5(3):215-226. doi:10.1016/j.jalz.2009.01.028
- Institute for Clinical and Economic Review. Report at a glance: Alzheimer’s disease. Published August 2021. Accessed August 16, 2022. https://icer.org/wp-content/uploads/2020/10/Alzheimers-Disease-RAAG_08052021_vFINAL.pdf
- Alzheimer’s Association, Centers for Disease Control and Prevention.Healthy Brain Initiative. State and local public health partnerships to address dementia: the 2018-2023 road map. Alzheimer’s Association; 2018. Accessed August 15, 2022. www.cdc.gov/aging/pdf/2018-2023-Road-Map-508.pdf
- Chen Y, Wilson L, Kornak J, et al. The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimer’s Dement. 2019;15(7):899-906. doi:10.1016/j.jalz.2019.03.015
- Pyenson B, Sawhney TG, Steffens C, et al. The real-world Medicare costs of Alzheimer disease: considerations for policy and care. J Manag Care Spec Pharm. 2019;25(7):800-809. doi:10.18553/jmcp.2019.25.7.800
- Rice DP, Fillit HM, Max W, Knopman DS, Lloyd JR, Duttagupta S. Prevalence, costs, and treatment of Alzheimer’s disease and related dementia: a managed care perspective. Am J Manag Care. 2001;7(8):809-818.
- Genworth. Cost of care survey. Accessed August 16, 2022. www.genworth.com/aging-and-you/finances/cost-of-care.html
- Kelly AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs in the last 5 years of life. Ann Intern Med. 2015;163(10):729-736. doi:10.7326/M15-0381
- Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B, Kuntz KM. Societal and family lifetime cost of dementia: implications for policy. J Am Geriatr Soc. 2017;65(10):2169-2175. doi:10.1111/jgs.15043
- Coinnews Media Group LLC. U.S. inflation calculator. Accessed August 16, 2022.
www.usinflationcalculator.com - Suehs BT, Shah SN, Davis CD, et al. Household members of persons with Alzheimer’s disease: health conditions, healthcare resource use, and healthcare costs. J Am Geriatr Soc. 2014;62(3):435-441. doi:10.1111/jgs.12694
- National Alliance for Caregiving. Research recommendations: dementia caregiving in the US. Published October 2017. Accessed August 16, 2022. www.caregiving.org/wp-content/uploads/2020/05/Dementia-Caregiving-Report-2017_Research-Recommendations_FINAL.pdf
- Upadhyay P, Weiner J. Issue brief: long-term care financing in the United States. Penn LDI. Published September 2019. Accessed August 16, 2022. https://ldi.upenn.edu/wpcontent/uploads/archive/pdf/LDI%20Issue%20Brief%202019%20Vol.%2023%20No.%201_7_0.pdf
- Sheehan OC, Haley WE, Howard VJ, Huang J, Rhodes JD, Roth DL. Stress, burden, and well-being in dementia and non-dementia caregivers: insights from the Caregiving Transitions Study. Gerontologist. 2021;61(5):670-679. doi:10.1093/geront/gnaa108
- Chen B, Cheng X, Streetman-Loy, B, Hudson MF, Jindal D, Hair N. Effect of care coordination on patients with Alzheimer disease and their caregivers. Am J Manag Care. 2020;26(11):e369-e375. doi:10.37765/ajmc.2020.88532
- Alzheimer’s Association, Centers for Disease Control and Prevention. A public health approach to Alzheimer’s and other dementias. Emory Rollins School of Public Health. Accessed August 15, 2022. www.cdc.gov/aging/aginginfo/pdfs/A-Public-Health-Approach-To-Alzheimers.pdf
- Alzheimer’s Association. Policy brief: early detection and diagnosis of Alzheimer’s dementia. Published August 2017. Accessed August 15, 2022. www.alz.org/media/Documents/policy-brief-early-detection-diagnosis-alzheimers.pdf
- Dubois B, Villain N, Fisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484-496. doi:10.1016/S1474-4422(21)00066-1
- National Institute on Aging. Research highlights: blood test can predict presence of beta-amyloid in the brain, new study finds. Published February 17, 2022. Accessed August 16, 2022. www.nia.nih.gov/news/blood-test-can-predict-presence-beta-amyloid-brain-new-study-finds
- Pais M, Martinez L, Ribeiro O, et al. Early diagnosis and treatment of Alzheimer’s disease: new definitions and challenges. Braz J Psychiatry. 2020;42(4):431-441. doi:10.1590/1516-4446-2019-0735
- Jack CR. World Alzheimer Report 2021: defining Alzheimer’s disease biologically. Alzheimer Disease International. Accessed August 16, 2022. www.alzint.org/u/World-Alzheimer-Report-2021.pdf
- Li Y, Schindler SE, Bollinger JG, et al. Validation of plasma amyloid-B 42/40 for detecting Alzheimer disease amyloid plaques. Neurology. 2022;98(7):e688-e699. doi:10.1212/WNL.0000000000003211
- New drug for Alzheimer’s disease – Aduhelm – update. Cure Alzheimer’s Fund. Published April 8, 2022. Accessed August 16, 2022. https://curealz.org/news-and-events/new-drug-for-alzheimers-disease-aduhelm/
- Centers for Medicare & Medicaid Services. National coverage determination: Beta amyloid positron tomography in dementia and neurodegenerative disease. Accessed August 16, 2022. www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=356
- Wimo A. World Alzheimer Report 2021: new challenges and opportunities in the diagnosis of dimentia. Alzheimer Disease International. Accessed August 16, 2022. www.alzint.org/u/World-Alzheimer-Report-2021.pdf
- Kirson NY, Desai U, Ristovska L, et al. Assessing the economic burden of Alzheimer’s disease patients first diagnosed by specialists. BMC Geriatr. 2016;16:138. doi:10.1186/s12877-016-0303-5
- Baumgart M, Snyder HM, Carillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718-726. doi:10.1016/j.jalz.2015.05.016
- Lin PJ, Yang, Z, Fillit HM, Cohen JT, Neumann PJ. Unintended benefits: the potential economic impact of addressing risk factors to prevent Alzheimer’s disease. Health Aff (Millwood). 2014;33(4):547-554. doi:10.1377/hlthaff.2013.1276
- Black CM, Fillit H, Xie L, et al. Economic burden, mortality, and institutionalization in patients newly diagnosed with Alzheimer’s disease. J Alzheimers Dis. 2018;61(1):185-193. doi:10.3233/JAD-170518
- Black CM, Lipton RB, Thiel E, Brouillette M, Khandker R. Relationship between treatment initiation and healthcare costs in Alzheimer’s disease. J Alzheimer’s Dis. 2019;68(4):1575-1585. doi:10.3233/JAD-180983.
- Green C, Handels R, Gustavsson A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: an open-source modeling framework. Alzheimers Dement. 2019;15(10):1309-1321. doi:10.1016/j.jalz.2019.05.004
- Bass DM, Judge KS, Maslow K, et al. Impact of the care coordination program “Partners in Dementia Care” on veterans’ hospital admissions and emergency department visits. Alzheimers Dement (N Y). 2015;1(1):13-22. doi:10.1016/j.trci.2015.03.003
- Lines LM, Ahaghotu C, Tilly J, Weiner JM. Care coordination for people with Alzheimer’s disease and related dementias: literature review. US Department of Health and Human Services. Published December 2013. Accessed August 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//44121/AlzCC.pdf
- United States Census Bureau. 2017 national population projections tables: main series (table 2). Revised October 8, 2021. Accessed August 16, 2022.www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html
- Liu JL, Hlavka JP, Hillestad R, Mattke S, Assessing the preparedness of the U.S. Health Care System Infrastructure for an Alzheimer’s treatment. RAND Corporation. Accessed August 16, 2022. www.rand.org/pubs/research_reports/RR2272.html
- Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s disease: evolving the care team for optimal patient management. Front Neurol. 2021;11:592302. doi:10.3389/fneur.2020.592302
- Gerontological Society of America. The Gerontological Society of America workgroup on cognitive impairment detection and earlier diagnosis: report and recommendations. Accessed August 16, 2022. www.geron.org/images/gsa/documents/gsaciworkgroup2015report.pdf
- Shim YW, Chua SS, Wong HC, Alwi S. Collaborative intervention between pharmacists and
physicians on elderly patients: a randomized controlled trial. Ther Clin Risk Manag. 2018;14:1115-1125. doi:10.2147/TCRM.S146218 - Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaun C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
- Desai U, Kirson NY, Ye W, Mehta NR, Wen J, Andrews JS. Trends in health service use and potentially avoidable hospitalizations before Alzheimer’s disease diagnosis: a matched, retrospective study of US Medicare beneficiaries. Alzheimers Dement (Amst). 2019;11:125-135. doi:10.1016/j.dadm.2018.12.005
- Lin PJ, Rane PB, Fillit HM, Cohen JT, Neuman PJ. O2-11-01: national estimates of potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related dementia. Alzheimers Dement. 2016;12(7S_Part 5):P253-P254. doi:10.1016/j.jalz.2016.06.454
- Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (N Y). 2021;7(1):e12179. doi:10.1002/trc2.12179
- Millan-Calenti JC, Lorenzo-Lopez L, Alonso-Bua, de Labra C, González-Abraldes I, Maseda A. Optimal nonpharmacological management of agitation in Alzheimer’s disease: challenges and solutions. Clin Interv Aging. 2016;11:175-184. doi:10.2147/CIA.S69484.
- Marasco RA. Current and evolving treatment strategies for the Alzheimer disease continuum. Am J Manag Care. 2020;26(8 Suppl):S167-S176. doi:10.37765/ajmc.2020.88481
- Zhang Q, Shultz JL, Aldridge GM, et al. COVID-19 case fatality and Alzheimer’s disease. J Alzheimers Dis. 2021;84(4):1447-1452. doi:10.3233/JAD-215161
- Matias-Guiu JA, Pytel V, Matias-Guiu J. Death rate due to COVID-19 in Alzheimer’s disease and frontotemporal dementia. J Alzheimers Dis. 2020;78(2):537-541. doi:10.3233.JAD-200940
- FDA grants accelerated approval for Alzheimer’s drug. News release. FDA; June 7, 2021. Accessed August 16, 2022. www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- Aduhelm. Prescribing information. Biogen; 2022. Accessed August 15, 2022. https://biogencdn.com/us/aduhelm-pi.pdf
- Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2021;17(4):696-701. doi:10.1002/alz.12213
- Three FDA advisers quit over agency approval of Aduhelm. Medical Xpress. Published June 14, 2021. Accessed August 16, 2022. https://medicalxpress.com/news/2021-06-fda-agency-aduhelm.html
- Thompson D. FDA defends approval of controversial Alzheimer’s drug. Medical Xpress. Published June 8, 2021. Accessed August 16, 2022. https://medicalxpress.com/news/2021-06-fda-defends-controversial-alzheimer-drug.html
- Toobin J. The road to Aduhelm: what one ex-FDA adviser called ‘probably the worst drug approval decision in recent US history’ for an Alzheimer’s treatment. CNN. Updated September 27, 2021. Accessed August 16, 2022. www.cnn.com/2021/09/26/politics/alzheimers-drug-aduhelm-fda-approval/index.html
- Heinrich AM, Schweitzer K. Alzheimer’s disease, Aduhelm, and the impact on Medicare. Milliman. Published February 14, 2022. Accessed August 16, 2022. https://us.milliman.com/en/insight/alzheimers-disease-aduhelm-and-the-impact-on-medicare
- Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. News release. Biogen; December 20, 2021. Accessed August 16, 2022. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients.
- Centers for Medicare & Medicaid Services. National coverage analysis: Monoclonal antibodies directed against amyloid for the treatment of Alzheimer’s disease. Accessed August 16, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305
- Sachs R. Understanding Medicare’s Aduhelm coverage decision. Health Affairs Forefront. January 12, 2022. Accessed July 13, 2022. https://petrieflom.law.harvard.edu/resources/article/understanding-medicares-aduhelm-coverage-decision
- Cohen J. CMS’s decision to restrict coverage of Aduhelm very unlikely to impact accelerated approvals in other classes. Forbes. Published May 2, 2022. Accessed August 16, 2022. www.forbes.com/sites/joshuacohen/2022/05/02/cmss-decision-to-restrict-coverage-of-aduhelm-very-unlikely-to-impact-other-accelerated-approvals/?sh=7b6176a3248f
- United Healthcare. United Healthcare Commercial Medical Benefit Drug Policy: Aduhelm (aducanumab-Avwa). Effective June 1, 2022. Accessed August 16, 2022. www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/aduhelm.pdf
