- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
The Current and Emerging Treatment Landscape for Chronic Cough
ABSTRACT
Cough serves a protective physiologic function as a vital defensive reflex preventing aspiration. However, exposure to viral infections or other triggers induces, in some individuals, a chronic cough (CC) that causes a significant symptomatic burden. Most cases of CC are due to conditions that respond to appropriate therapeutic trials (upper airway cough syndrome; asthma; reflux). Unfortunately, a significant subgroup of patients will have refractory CC, which does not respond to treatment of known underlying causes of CC. Currently, available therapeutic options for refractory CC are inadequate due to limited efficacy and frequently intolerable adverse effects. Current professional society guideline recommendations are discussed, and a promising pipeline of antitussive drugs in development is introduced, including purinergic 2X3 receptor antagonists, neurokinin-1 receptor antagonists, oral mixed ĸ-opioid receptor agonists/µ-opioid receptor antagonists, and voltage-gated sodium channel blockers.
Am J Manag Care. 2022;28(suppl 10):S159-S165. https://doi.org/10.37765/ajmc.2022.89244
Introduction
The most frequent cause of cough is the common cold (or upper respiratory tract infection [URTI]).1 The cough has a protective physiologic function; however, chronic cough (CC) often prompts patients to seek medical care. The etiology of cough in children and adults can differ, and many patients experience treatment-refractory cough even when a potential underlying cause has been identified.2,3 CC is often attributed to unexplained postinfectious cough or pulmonary disorders (eg, asthma, chronic obstructive pulmonary disease, postnasal drip, idiopathic pulmonary fibrosis, lung cancer).
CC may cause sleep disturbances, chest pain, fatigue, and nausea that impairs quality of life (QOL).4-7 People often find CC embarrassing if it causes urinary incontinence or affects mood.8 A 2018 National Health and Wellness Survey analysis compared approximately 5% of people with CC with respondents who did not have CC. The study found that people with CC had experienced severe anxiety and depression in the past 2 weeks, impaired productivity, poor sleep quality, and daytime sleepiness more frequently than those without CC. Another study confirmed that depression is commonly seen as well.9 Respondents with CC also presented to the emergency department and were hospitalized more often than those without CC.10
Evaluation and Management of Chronic Cough
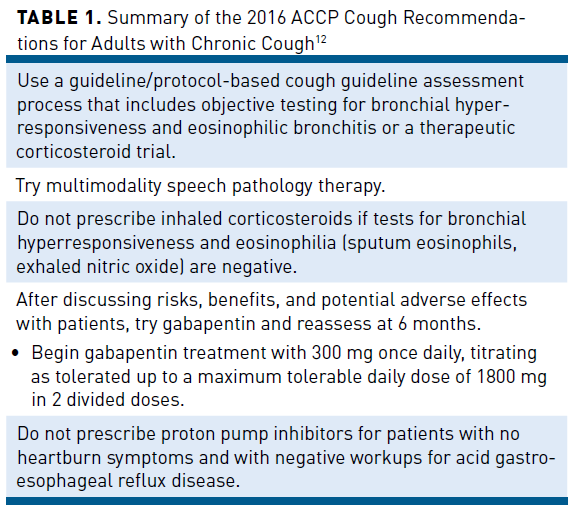
Two recently published guidelines offer suggestions and algorithms for CC evaluation and treatment.11,12 The 2016 American College of Chest Physicians (ACCP) guidelines classify cough as unexplained CC if it persists for longer than 8 weeks with no apparent etiology after evaluation and supervised therapeutic trial(s) in compliance with published best-practice guidelines. Refractory chronic cough (RCC) is the more commonly used term that refers to CC not responding to therapeutic trials aimed at known reversible causes of cough.13 The approach outlined in the guidelines is 3-fold: adequate assessment, investigation, and therapy (Table 112).11,12
The European Respiratory Society (ERS) released an updated version of its guidelines on the diagnosis and treatment of CC in adults and children in 2020.13 The expert panel indicated that a better understanding of some individuals’ exquisite sensitivity to external stimuli (eg, cold air, perfumes, smoke, bleach) provides a physiological explanation for cough based on vagal afferent hypersensitivity. The ERS guidelines indicate that cough-variant asthma and eosinophilic bronchitis respond to anti-inflammatory therapy. Nonacid reflux may respond to promotility agents rather than antacid medications. The ERS guidelines also discuss the benefit of neuromodulator therapies. Unlike the ACCP guidelines, the ERS guidelines recommend low-dose morphine over gabapentin in a subset of patients with RCC because of adverse effects (AEs).13
Over-the-Counter Options
The choice of medication varies based on the etiology of a person’s cough, and treatment plans should be individualized. The most accessible options are available over the counter (OTC) and may be the most familiar. Because they are readily available, inexpensive, and have relatively few AEs, people commonly use nonprescription options for self-treatment of cough; however, none of these agents are indicated for CC. Clinical guidelines also do not recommend the use of nonprescription agents as robust evidence for their efficacy is lacking; however, because they are so commonly used, a brief review of the agents is discussed. If an individual opts to use a nonprescription treatment for their CC, they should be instructed to follow the drug facts label for dosing, what to expect in terms of AEs, and when further evaluation is required. Generally, nonprescription products should not be used for extended periods, so referral for an additional workup to guide further treatment decisions is necessary.
Guaifenesin
Guaifenesin, extracted from guaiac tree bark, is the only expectorant approved by the FDA. Its mechanism of action is unclear, but it may influence the cholinergic innervation of airway mucous glands.14 Although it is FDA approved, the body of evidence supporting its effect on ciliary motility or mucociliary clearance, as well as its efficacy as either an expectorant or antitussive, is conflicting.15,16
One record of a study in 1977 utilizing 20 mg guaifenesin 4 times daily for 3 days found that 75% of guaifenesin-treated patients had reduced subjective cough severity compared with 31% in the placebo group.17,18 A later study in 1982 utilizing a higher dose (480 mg every 6 hours for 30 hours) found no significant reduction in objective cough frequency or subjective cough severity in patients with URTI with the use of guaifenesin.18,19 Patients with productive cough reported reduced sputum viscosity, but its clinical impact was questionable. In 2003, a study in patients with URTI found that guaifenesin inhibited cough reflex sensitivity.20 Additional studies are needed as available data provide no conclusive evidence about the antitussive effect of guaifenesin.
Dextromethorphan
By sales, dextromethorphan is the most widely used OTC antitussive drug in the United States, and approximately 85% to 90% of OTC cough medicines contain dextromethorphan.21 The FDA approved dextromethorphan for cough in 1958 based on a few clinical trials that demonstrated modest benefit, though they did not include placebo arms or use validated outcome measures.22,23 It was not until 1996 that a clinical study reported on the effect of dextromethorphan on cough.24 Although this small, placebo-controlled trial was conducted almost 50 years after the approval of dextromethorphan and addressed many of the limitations present in previous studies, investigators found no clinical benefit to its use.25
In 2007, public health officials asked the FDA to review the efficacy and safety of dextromethorphan as an OTC antitussive in response to reports of accidental overdoses and dextromethorphan abuse, particularly in children.26,27 Dextromethorphan has euphoric, hallucinogenic, and dissociative properties when used at supratherapeutic doses. The FDA completed a review in 2010, and in light of what appeared to be a plateauing number of cases, its Advisory Committee members recommended an abuse mitigation plan.21 Since the implementation of the abuse mitigation plan directed at children and adolescents by the FDA, reports of abuse have decreased by approximately 35%.21
First-generation Antihistamines
Histamine H1-receptor antagonists can combine with and stabilize the inactive form of the H1-receptor, shifting the equilibrium toward the inactive state.28 In addition, some H1-antihistamines can inhibit muscarinic, α-adrenergic, and serotonin receptors as well as some ion channels. Animal studies suggest H1-antihistamines have antitussive activity, but induced cough studies in healthy human volunteers have yielded mixed results.29
Diphenhydramine
Diphenhydramine is a commonly used oral first-generation antihistamine. The FDA classifies it as an antitussive in its OTC monograph despite sparse evidence supporting its efficacy. The ACCP guidelines recommend combining a first-generation H1-antihistamine and a decongestant for CC due to upper airway cough syndrome (ie, postnasal drip syndrome) and acute cough due to the common cold.12
A small, randomized, double-blind study (N = 22) conducted in 2015 compared a diphenhydramine (25 mg) and phenylephrine (10 mg)-containing multicomponent syrup with a 30-mg dextromethorphan-containing syrup and placebo to assess cough reflex sensitivity in otherwise healthy individuals with acute URTI.30 The researchers administered a capsaicin challenge on 3 separate days to coincide with peak blood concentrations of diphenhydramine. The end point was the capsaicin-inducing 5 or more coughs (C5). There was a significant (P <.01) increase in mean log C5 for the diphenhydramine-containing medication compared with placebo but not for dextromethorphan versus placebo. Significant (P = .0024) between-group differences were observed with lower log C5 differences in the dextromethorphan and placebo groups. AEs included slight drowsiness after receipt of diphenhydramine (n = 5), dextromethorphan (n = 2), and placebo (n = 1). In the dextromethorphan arm, participants reported slight nausea (n = 2) and dry mouth (n = 1).30
Chlorpheniramine
Chlorpheniramine is a first-generation histamine and is primarily used to treat allergic symptoms as it facilitates mucociliary clearance and reduces mucus production. In addition, chlorpheniramine is used to treat cough, though the literature contains little evidence of its effectiveness; recent studies are challenging to find.31 A study conducted between March 2014 and June 2016 enrolled 218 patients hospitalized with CC or rhinitis/sinusitis who received chlorpheniramine combined with guaiacol glyceryl ether/dextromethorphan hydrobromide.32 The researchers divided patients into 2 groups based on the magnitude of reduction in cough frequency (effective and ineffective). In follow-up visits on day 15 after discharge, 114 participants (52.29%) reported a cough frequency reduced by at least half, improved nasal-related performance, and reduced nasal mucus. The rate of chronic rhinitis/sinusitis was similar between the groups. The researchers concluded that, with a 52% effectiveness rate and no statistical difference between groups, the therapeutic efficacy of chlorpheniramine could not be proven for cough.31 Chlorpheniramine is also available in spray and intranasal gel forms.31
Prescription Options
Clinicians should select targeted therapy based on suspected underlying etiologies of cough, including upper airway cough syndrome, asthma, or gastroesophageal reflux disease. Most current prescription treatments for RCC act on the central nervous system (CNS) to interfere with the aberrant cough response.33-36 Despite some evidence of effectiveness in CC, the majority of these agents do not elicit responses in patients or are associated with intolerable AEs.13
Benzonatate
Researchers determined that benzonatate inhibits pulmonary stretch receptors and is thus a peripherally acting drug.37-40 The apparent mechanism of action for the antitussive effect associated with benzonatate is peripheral anesthesia and inhibition of afferent vagal fibers from pulmonary stretch receptors located in the bronchial tree.38 Its onset of action is 15 to 20 minutes, and its duration of action is 3 to 8 hours.37 Benzonatate (Tessalon Perles) was the last antitussive approved by FDA in 1958 based on evidence from 2 small studies.37 Traditionally, clinicians considered the AE profile of benzonatate to be mild. However, in 2010 the FDA issued a drug safety communication after receiving reports of death due to accidental overdose of benzonatate by children younger than 10 years.41
A 2009 study was designed to investigate the effect of benzonatate on capsaicin-induced cough in nonsmoking patients with acute URTI. Researchers found that benzonatate had no antitussive effect when administered alone; however, it was effective when administered in combination with guaifenesin.42 The recommended dose of benzonatate for adults and children older than 10 years is 100 mg to 200 mg by mouth every 8 hours as needed, with a maximum daily dose of 600 mg.37 Patients should be instructed to swallow the benzonatate capsule (perle) whole, without chewing, to avoid upper airway/pharyngeal anesthetic effects.
Neuromodulators
The ACCP and ERS guidelines indicate that 3 drug classes fall into the neuromodulator category: opiates, analogues of the neurotransmitter gamma-aminobutyric acid, and tricyclic antidepressants (TCAs).12,13 When the ACCP guidelines were published in 2016, only 1 randomized controlled trial was available for each category.12
Opiates
Clinicians have used opiates for many years for cough, usually prescribing low-dose morphine or codeine (methylmorphine). Low-dose morphine acts on µ-opioid receptors in the CNS to inhibit cough reflex pathways.34,43 When neuromodulatory medications are started, the ERS guidelines recommend a trial of low-dose, slow-release morphine (5 mg to 10 mg twice daily) in adults with RCC. In contrast, the ACCP guidelines recommend gabapentin and do not recommend morphine.12 Common AEs include constipation and drowsiness in patients receiving morphine.13
The ERS guidelines do not recommend codeine unless it is the only available option, citing interindividual genetic variability in drug metabolism (CYP2D6) that makes treatment response and AE profile less predictable than other medications, particularly in children.13 Some experts consider codeine-containing products no more effective than dextromethorphan.44 Codeine-containing products should be used with caution in patients with a history of addiction and should be avoided in patients with asthma, respiratory depression, and chronic obstructive pulmonary disease.45 The Drug Enforcement Agency classifies codeine-containing products for cough as Schedule V narcotics if they contain less than 200 mg codeine/100 mL. In 2018, the FDA limited use of codeine for cough indications to adults aged 18 years and older, indicating risks exceeded potential benefits in children; prescription cough and cold medications now carry a boxed warning.46 In 30 states and Washington DC, Schedule V cough syrups can be purchased without a prescription.47
Other Neuromodulators
Neuromodulators appear to reduce neuronal overexcitability, which is common to both pain and CC.48 Gabapentin and pregabalin are known to act on the α2δ subunit of the voltage-dependent calcium channel in the CNS to modulate Ca2+ ion influx and inhibit neurotransmitter release in the CNS.49,50
Australian researchers performed a randomized, double-blind, placebo-controlled trial (N = 62) in an outpatient setting to assess gabapentin (n = 32) for 10 weeks in adults with RCC compared with placebo (n = 30).51 The primary end point was a change in the cough-specific QOL comparing Leicester Cough Questionnaire (LCQ) scores at baseline and after 8 weeks of treatment. Gabapentin significantly improved cough-specific QOL compared with placebo. Overall, gabapentin was effective and well tolerated; AEs were reported by 10 participants (31%) who received gabapentin and 3 participants in the placebo arm, with nausea and fatigue being the most common.51
Researchers from the Cleveland Clinic evaluated the short- and long-term effects of TCAs and gabapentin when used to treat RCC.48 They used a prospective cohort design, enrolling participants between July 2016 and March 2017 following formal workup and clinical evaluation. Participants received either a TCA (amitriptyline or nortriptyline) or gabapentin. Twenty-eight patients completed 37 neuromodulator trials, with statistically significant improvement in LCQ scores in the gabapentin group at 2 months (2.48 points, P <.01) and 6 months (5.40 points, P = .01) compared with baseline. Among participants taking TCAs, LCQ score improvements at 2 months of treatment (3.46 points, P <.01) were statistically significant. Most participants discontinued treatment after 2 months, with tachyphylaxis being the most common reason; these patients were excluded from analysis at 6 months. Participants in the TCA arms were more likely to discontinue treatment than those in the gabapentin arm.48
Speech Language Pathology Treatment
As noted in Table 112, the ACCP guidelines recommend physiotherapy/speech and language therapy (cough control therapy) in adults with RCC.12 Components of speech pathology treatment (SPT) include education about the nature of CC, strategies to control it, psychoeducational counseling, and vocal hygiene education to reduce laryngeal irritation.52 SPT has been shown to significantly reduce subjective cough score; coverage for consultation is dependent on a patient’s specific insurance plan.52,53
A randomized, placebo-controlled trial enrolled 40 participants to receive either SPT and pregabalin 300 mg daily or SPT plus placebo.36 Primary outcome measures were cough frequency, cough severity using a visual analog scale (VAS), and cough-related QOL using the LCQ. Both groups experienced improved cough severity, cough frequency, and cough-related QOL. Participants in the combined SPT/pregabalinarm were more likely tohave an improvement in LCQ and cough severity VAS than those who received SPT alone. Cough frequency, however, was similar in both groups. Participants’ symptoms did not deteriorate after pregabalin was discontinued. Combined SPT and pregabalin was associated with a median capsaicin cough sensitivity improvement from 15.7 to 47.5 mM, whereas SPT alone was associated with a median capsaicin cough sensitivity improvement from 3.92 to 15.7 mM.36
Emerging Targeted Therapy
Due to the lack of efficacy with traditional cough therapies and a better understanding of the pathophysiology of cough, there has been increased development and investigation of new treatments. These newer agents have various mechanisms of action that target specific receptors or channels in the peripheral sensory neuron to reduce CNS AEs and control hypersensitivity while preserving the protective cough response. Current clinical trials are assessing dose, administration, safety, and efficacy.
Purinergic 2X3 Receptor Antagonists
Researchers developed the first-in-class selective purinergic 2X3 (P2X3) receptor antagonist with acceptable pharmacodynamic, pharmacokinetic, and in vivo activity roughly 10 years ago.55 P2X3 receptors are located on sensory nerve fibers, especially C fibers, in peripheral tissues, including the airway lining, but not in the CNS. Airway lining cells can release chemical stimuli, including adenosine triphosphate (ATP), if airway inflammation, irritation, or mechanical stress/injury is present. Extracellular binding of ATP to P2X3 receptors on C fibers in the airway can create an afferent signal stimulating the brain stem to produce cough. Once activated by ATP, P2X3 receptors can sensitize vagal afferents and heighten sensory nerve reflexes.54
Inhibiting the ability of extracellular ATP to bind to P2X3 receptors appears to reduce sensory nerve activation and, subsequently, cough. Although sensory nerves expressing P2X receptors appear to contribute to coughing, P2X receptors, specifically P2X2/3, are also involved in taste perception.54
Gefapixant
Gefapixant is a selective P2X3 receptor antagonist that has been approved in Japan but remains investigational in the United States.55 A cough challenge study in patients with CC and healthy volunteers examined the specificity of gefapixant on pathological cough reflex using 4 different inhalation agents, differentiating between ATP-mediated cough and irritant-induced cough.56 Gefapixant had no effect on cough response induced by capsaicin and citric acid though it significantly reduced ATP- and distilled water-provoked coughs.
In early studies, gefapixant 600 mg twice daily was associated with a 75% reduction in 24-hour ambulatory cough counts. At this dose, 88% of participants receiving gefapixant reported dysgeusia, which appeared to be related to gefapixant inhibiting the P2X2/3 heterotrimer involved in taste sensation.57 Dose-evaluation studies demonstrated 50 mg gefapixant twice daily significantly reduced objective cough frequency, with 45% of participants reporting dysgeusia.58,59
The phase 3 multinational, randomized, double-blind, placebo-controlled COUGH-1 (N = 730) and COUGH-2 (N = 1314) clinical trials were the first companion studies to be conducted in patients with RCC.60 COUGH-1 had a 12-week treatment period and a 40-week extension period, while COUGH-2 had a 24-week treatment period and a 28-week extension period. Both studies evaluated the efficacy and safety of gefapixant in reducing cough frequency in adult participants with RCC. Participants received a regimen of gefapixant 45 mg twice daily, gefapixant 15 mg twice daily, or placebo. The primary efficacy outcome was 24-hour cough frequency at baseline, week 12, and week 24 as recorded by an ambulatory digital audio recording device. Secondary end points included the number of awake coughs per hour and subjective outcomes, including changes from baseline in the LCQ total score.60
Gefapixant 45 mg twice daily significantly reduced the 24-hour cough count at week 12 in COUGH-1 and at week 24 in COUGH-2 compared with placebo, despite a large placebo effect.60 The most common AEs were related to taste disturbance and included ageusia (4.9% in COUGH-1 and 6.5% in COUGH-2), dysgeusia (16.2% in COUGH-1 and 21.1% in COUGH-2), hypergeusia (0.4% in COUGH-1 and 0.5% in COUGH-2), hypogeusia (2.6% in COUGH-1 and 6.1% in COUGH-2), and taste disorder (3.8% in COUGH-1 and 3.5% in COUGH-2).60
The manufacturer applied for licensure of gefapixant in December 2021. In January 2022, the FDA issued a Complete Response Letter asking for additional information related to efficacy measurement. The request was not related to the safety; further research will determine its potential role in management.61
BLU-5937
BLU-5937 is a selective P2X3 receptor antagonist that significantly reduced ATP-induced enhancement of citric acid-induced coughs in preclinical trials.62 This finding was achieved at plasma concentrations known to block the homotrimeric P2X3 receptors and was also 50 times lower than the concentration required to antagonize the heterotrimeric P2X2/3 receptor. The selectivity of BLU-5937 reduces the likelihood of taste disturbance. At concentrations 30 times higher than the half-maximal inhibitory concentration for P2X3, no taste disturbances were observed in an animal model.62
A phase 2a proof-of-concept, randomized, crossover, dose-escalation study (RELIEF, N = 69) evaluated the efficacy and safety of BLU-5937 in participants with RCC.63,64 An ambulatory cough recorder was used to measure cough frequency, which participants in both groups reported to be a significant burden on their QOL. Those treated with all BLU-5937 doses (25 mg, 50 mg, 100 mg, and 200 mg twice daily) experienced numerical reductions in cough frequency compared with placebo in the intent-to-treat (ITT) population; the reductions did not reach statistical significance at any dose. BLU-5937 reduced awake and 24-hour cough counts in patients with CC with higher cough counts (32.4 coughs/hour as a predetermined threshold) compared with placebo.63,64
Preliminary results from the phase 2b SOOTHE study (N = 249 adults with a cough frequency ≥25 coughs/hour) were released in September 2021. There was a 34% placebo-adjusted reduction in 24-hour cough frequency in participants treated with BLU-5937 50 mg or 200 mg twice daily on day 28; the 12.5-mg dose did not reach statistical significance. Though taste disturbance occurred in fewer than 6.5% of participants, the AE profile of BLU-5937 was similar to placebo with no treatment-related serious AEs reported.65
Eliapixant
Eliapixant is a potent, selective P2X3 receptor antagonist with an improved AE profile. In a phase 2b placebo-controlled, randomized, double-blind, parallel-group, dose-finding trial (PAGANINI), patients with RCC (N >300) received 25 mg, 75 mg, or 150 mg of eliapixant, or placebo tablets twice daily for 12 weeks.66 Oral eliapixant doses at or above 50 mg reduced 24-hour cough counts by 30% compared with placebo at week 12. Taste-related AEs were reported by 24% of participants treated with the 150-mg dose and were far less common in participants receiving the lower doses.67 However, in February 2022, the clinical development program for eliapixant was terminated by the company due to a risk/benefit analysis.68
Sivopixant
Researchers are currently investigating the highly selective P2X3 receptor antagonist sivopixant (S-600918) for the treatment of CC.69 A phase 2a study in patients with CC found 24-hour cough counts fell by 30.9% compared with placebo. Sivopixant is a more selective drug, and taste-related AEs are less likely than those associated with less selective P2X3 receptor antagonists.69
Neurokinin-1 Receptor Antagonist: Orvepitant
A recent open-label phase 2a (VOLCANO-1) study showed the efficacy of a neurokinin (NK)-1 antagonist, orvepitant, in significantly reducing 24-hour cough count in patients with CC.70 The subsequent phase 2b randomized, placebo-controlled study showed an insignificant reduction in 24-hour cough counts at 12 weeks despite a significant reduction in 3 different patient-reported outcomes in those with CC.70 With these mixed results, it remains to be seen what role, if any, NK-1 receptor antagonists will play in the treatment of CC in the future. Orvepitant is planned for evaluation in a study of CC associated with idiopathic pulmonary fibrosis (IPF).71
Voltage-Gated Sodium Channel Blockers
Voltage-gated sodium channels (NaVs, especially NaV1.7, 1.8, and 1.9 subtypes) initiate action potentials needed for conduction under any stimulus and are now a neural target for CC. NaV1.7, 1.8, and 1.9 subtypes are predominantly expressed in airway cough-triggering nerves. A concern is that suppressing airway cough-triggering nerves may also block beneficial sensations and neuronal reflex behavior. The concept is that new antitussive drugs would show benefit from targeting peripheral airway nociceptors without inhibiting the protective cough reflex. These entities are now in phase 1 and early phase 2 development.72
Nalbuphine
Extended-release nalbuphine, an oral mixed ĸ-opioid receptor agonist and µ-opioid receptor antagonist, is being investigated for cough. This drug has been FDA approved and marketed as an injectable analgesic for over 20 years, but it is not classified as a controlled substance. The ĸ- and µ-opioid receptors are important mediators of itch, cough, and certain movement disorders.73,74 Nalbuphine antagonizes µ-opioid receptors and may reduce the risk of µ-opioid agonist abuse. The FDA has granted Fast Track designation for nalbuphine for the reduction of moderate to severe pruritus in patients with prurigo nodularis.74
The phase 2 Cough And NALbuphine (CANAL) trial is being conducted in patients with IPF experiencing CC, with positive interim analysis results reported in early 2022.74 Due to the strength and consistency of the data, there was a move to end screening, conclude enrollment, and stop the trial. The final accrual is expected to be 40 participants. Based on interim data (N = 26), oral nalbuphine was associated with a 52% placebo-adjusted reduction in daytime cough events (P <.0001, conditional power 100%). Patient-reported outcome measure changes also signaled an improvement in daytime cough frequency. The safety profile was consistent with previous studies. As nalbuphine has been marketed for years, its safety profile is well established; no new safety signals were reported. Common adverse reactions associated with nalbuphine include sedation, sweating or clammy feelings, nausea/vomiting, dizziness/vertigo, dry mouth, and headache.75
Conclusions
Traditionally, clinicians have had to treat RCC with primarily centrally acting antitussives that are largely ineffective and are associated with intolerable AEs. Until effective agents are available for clinical use, clinicians should maximize guidance from existing guidelines to help patients with RCC. With many investigational compounds currently in clinical trials, a peripherally acting antitussive for treatment-refractory CC is eagerly anticipated. Several classes of drugs are advancing through clinical trials with promising results as there is a huge need for proper treatment for CC. n
Author affiliations: Peter Dicpinigaitis, MD, is Professor of Medicine, Albert Einstein College of Medicine, Division of Critical Care Medicine, Montefiore Medical Center; and Director, Montefiore Cough Center, Bronx, NY.
Funding source: This activity is supported by an educational grant from Merck Sharp & Dohme Corp.
Author disclosure: Dr Dicpinigaitis has the following relevant financial relationships with commercial interests to disclose:
Consultant: Bayer HealthCare, Bellus, Chiesi, Merck Sharp & Dohme Corp, Shionogi
Authorship information: Concept and design; drafting of the manuscript; and critical revision of the manuscript for important intellectual content.
Address correspondence to: dicipini@montefiore.org
Medical writing and editorial support provided by: Jeannette Y. Wick, RPh, MBA, FASB
REFERENCES
- de Jongste JC, Shields MD. Cough. 2: chronic cough in children. Thorax. 2003;58(11):998-1003. doi:10.1136/thorax.58.11.998
- Birring SS, Murphy AC, Scullion JE, Brightling CE, Browning M, Pavord ID. Idiopathic chronic cough and organ-specific autoimmune diseases: a case-control study. Respir Med. 2004;98(3):242-246. doi:10.1016/j.rmed.2003.10.005
- Everett CF, Kastelik JA, Thompson RH, Morice AH. Chronic persistent cough in the community:
a questionnaire survey. Cough. 2007;3:5. doi:10.1186/1745-9974-3-5 - French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657-1661. doi:10.1001/archinte.158.15.1657
- Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339-343. doi:10.1136/thorax.58.4.339
- Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung. 2008;186(suppl 1):S55-S58. doi:10.1007/s00408-007-9034-x
- Yousaf N, Lee KK, Jayaraman B, Pavord ID, Birring SS. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough. 2011;7(1):4. doi:10.1186/1745-9974-7-4
- Dicpinigaitis PV. Prevalence of stress urinary incontinence in women presenting for evaluation of chronic cough. ERJ Open Res. 2021;7(1):00012-2021. doi:10.1183/23120541.00012-2021
- Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest. 2006;130(6):1839-1843. doi:10.1378/chest.130.6.1839
- Meltzer EO, Zeiger RS, Dicpinigaitis P, et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract. 2021;9(11):4037-4044.e2. doi:10.1016/j.jaip.2021.07.022
- Irwin RS, French CT, Lewis SZ, Diekemper RL, Gold PM; CHEST Expert Cough Panel. Overview of the management of cough: CHEST Guideline and Expert Panel Report. Chest. 2014;146(4):885-889. doi:10.1378/chest.14-1485
- Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST Guideline and Expert Panel Report. Chest. 2016;149(1):27-44. doi:10.1378/chest.15-1496
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children [published correction appears in Eur Respir J. 2020;19;56(5)]. Eur Respir J. 2020;55(1):1901136. doi:10.1183/13993003.01136-2019C14
- Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respir Care. 2007;52(7):859-865.
- Sisson JH, Yonkers AJ, Waldman RH. Effects of guaifenesin on nasal mucociliary clearance and ciliary beat frequency in healthy volunteers. Chest. 1995;107(3):747-751. doi:10.1378/chest.107.3.747
- Yeates DB, Cohen VR, Davis AL, et al. Effect of glyceryl guaiacolate on bronchial clearance in patients with chronic bronchitis. Am Rev Respir Dis. 1977;115(suppl):182.
- Robinson RE, Cummings WB, Deffenbaugh ER. Effectiveness of guaifenesin as an expectorant: a cooperative double-blind study. Curr Ther Res. 1977;22:284-296.
- Schroeder K, Fahey T. Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ. 2002;324(7333):329. doi:10.1136/bmj.324.7333.329
- Kuhn JJ, Hendley JO, Adams KF, Clark JW, Gwaltney JM Jr. Antitussive effect of guaifenesin in young adults with natural colds. objective and subjective assessment. Chest. 1982;82(6):713-718. doi:10.1378/chest.82.6.713
- Dicpinigaitis PV, Gayle YE. Effect of guaifenesin on cough reflex sensitivity. Chest. 2003;124(6):2178-2181. doi:10.1378/chest.124.6.2178
- Spangler DC, Loyd CM, Skor EE. Dextromethorphan: a case study on addressing abuse of a safe and effective drug. Subst Abuse Treat Prev Policy. 2016;11(1):22. doi:10.1186/s13011-016-0067-0
- Cass LJ, Frederik WS, Andosca JB. Quantitative comparison of dextromethorphan hydrobromide and codeine. Am J Med Sci. 1954;227(3):291-296.
- Ralph N. Evaluation of a new cough suppressant. Am J Med Sci. 1954;227(3):297-303.
- Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. Evaluation of antitussive agents in man. Pulm Pharmacol. 1996;9(5-6):299-308. doi:10.1006/pulp.1996.0039
- Lee PCL, Jawad MS, Eccles R. Antitussive efficacy of dextromethorphan in cough associated with acute upper respiratory tract infection. J Pharm Pharmacol. 2000;52(9):1137-1142. doi:10.1211/0022357001774903
- Ritter D, Ouellette L, Sheets JD, et al. “Robo-tripping”: dextromethorphan toxicity and abuse. Am J Emerg Med. 2020;38(4):839-841. doi:10.1016/j.ajem.2019.10.001
- Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar—pediatric cough and cold medications. N Engl J Med. 2007;357(23):2321-2324. doi:10.1056/NEJMp0707400
- Monczor F, Fernandez N, Fitzsimons CP, Shayo C, Davio C. Antihistaminergics and inverse agonism: potential therapeutic applications. Eur J Pharmacol. 2013;715(1-3):26-32. doi:10.1016/j.ejphar.2013.06.027
- Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R243-R251. doi:10.1152/ajpregu.00184.2008
- Dicpinigaitis PV, Dhar S, Johnson A, Gayle Y, Brew J, Caparros-Wanderley W. Inhibition of cough reflex sensitivity by diphenhydramine during acute viral respiratory tract infection. Int J Clin Pharm. 2015;37(3):471-474. doi:10.1007/s11096-015-0081-8
- Rizvi SAA, Ferrer G, Khawaja UA, Sanchez-Gonzalez MA. Chlorpheniramine, an old drug with new potential clinical applications: a comprehensive review of the literature [published online ahead of print, June 1, 2022]. Curr Rev Clin Exp Pharmacol. 2022;10.2174/2772432817666220601162006. doi:10.2174/2772432817666220601162006
- Du F. Therapeutic effect of chlorpheniramine in treating upper airway cough syndrome (UACS) and chronic rhinitis/sinusitis. Pak J Pharm Sci. 2018;31(4(Special)):1679-1682.
- Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope. 2006;116(12):2108-2112. doi:10.1097/01.mlg.0000244377.60334.e3
- Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175(4):312-315. doi:10.1164/rccm.200607-892OC
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589. doi:10.1016/S0140-6736(12)60776-4
- Vertigan AE, Kapela SL, Ryan NM, Birring SS, McElduff P, Gibson PG. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648. doi:10.1378/chest.15-1271
- Tessalon. Prescribing information. Pfizer Inc; 2010. Accessed August 16, 2022. cdn.pfizer.com/pfizercom/products/uspi_tessalon.pdf
- Dicpinigaitis PV, Morice AH, Birring SS, et al. Antitussive drugs—past, present, and future. Pharmacol Rev. 2014;66(2):468-512. doi:10.1124/pr.111.005116
- Bucher K. Tessalon, ein hustenstillendes Mittel von neuartigem Wirkungsmechanismus [New effect mechanism of the antitussive drug tessalon]. Schweiz Med Wochenschr. 1956;86(4):94-96.
- Gregoire F, Thibaudeau Y, Comeau M. The treatment of cough by a non-narcotic antitussive. Can Med Assoc J. 1958;79(3):180-184.
- FDA Drug Safety Communication: death resulting from overdose after accidental ingestion of Tessalon (benzonatate) by children under 10 years of age. US FDA. Published December 14, 2010. Updated August 3, 2017. Accessed August 15, 2022. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-death-resulting-overdose-after-accidental-ingestion-tessalon
- Dicpinigaitis PV, Gayle YE, Solomon G, Gilbert RD. Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med. 2009;103(6):902-906. doi:10.1016/j.rmed.2008.12.008
- Adcock JJ, Douglas GJ, Garabette M, et al. RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol. 2003;138(3):407-416. doi:10.1038/sj.bjp.0705056
- Dicpinigaitis PV. Clinical perspective - cough: an unmet need. Curr Opin Pharmacol. 2015;22:24-28. doi:10.1016/j.coph.2015.03.001
- Tietze KJ. Chapter 12: Cough. In: Krinsky D, Berardi R, Ferreri S, et al, eds. Handbook of Nonprescription Drugs. 20th ed. American Pharmacists Association; 2021.
- FDA Drug Safety Communication: FDA requires labeling changes for prescription opioid cough and cold medicines to limit their use to adults 18 years and older. US FDA. Published January 22, 2018. Accessed August 15, 2022. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-labeling-changes-prescription-opioid-cough-and-cold
- The 2020 Survey of Pharmacy Law. National Association of Boards of Pharmacy. Published 2020. Accessed August 16, 2022. nabp.pharmacy/resources/publications/survey-of-pharmacy-law/
- Bowen AJ, Nowacki AS, Contrera K, et al. Short- and long-term effects of neuromodulators for unexplained chronic cough. Otolaryngol Head Neck Surg. 2018;159(3):508-515. doi:10.1177/0194599818768517
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission [published correction appears in Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82. doi:10.1016/j.tips.2006.12.006
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol. 2002;449(3):221-228. doi:10.1016/s0014-2999(02)02044-7
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589. doi:10.1016/S0140-6736(12)60776-4
- Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Efficacy of speech pathology management for chronic cough: a randomised placebo-controlled trial of treatment efficacy. Thorax. 2006;61(12):1065-1069. doi:10.1136/thx.2006.064337
- Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax. 2017;72(2):129-136. doi:10.1136/thoraxjnl-2016-208843
- North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83(4):759-769. doi:10.1124/mol.112.083758
- Merck provides US and Japan regulatory update for gefapixant. News release. Merck; January 24, 2022. Accessed August 16, 2022. www.merck.com/news/merck-provides-u-s-and-japan-regulatory-update-for-gefapixant/
- Morice AH, Kitt MM, Ford AP, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1):1900439. doi:10.1183/13993003.00439-2019
- Smith JA, Kitt MM, Butera P, et al. Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J. 2020;55(3):1901615. doi:10.1183/13993003.01615-2019
- Abdulqawi R, Dockry R, Holt K, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198-1205. doi:10.1016/S0140-6736(14)61255-1
- U.S. FDA accepts Merck’s gefapixant New Drug Application for review. News release. Merck; March 1, 2021. Accessed August 16, 2022. www.merck.com/news/u-s-fda-accepts-mercks-gefapixant-new-drug-application-for-review/
- McGarvey LP, Birring SS, Morice AH, et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022;399(10328):909-923. doi:10.1016/S0140-6736(21)02348-5
- FDA issues Complete Response Letter for Merck’s chronic cough treatment. Pharmaceutical Technology. Published January 25, 2022. Accessed August 16, 2022. www.pharmaceutical-technology.com/news/fda-merck-chronic-cough/
- Garceau D, Chauret N. BLU-5937: a selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther. 2019;56:56-62. doi:10.1016/j.pupt.2019.03.007
- Smith J, Morice AH, Birring SS, et al. Improvements in cough frequency over 24 hours with BLU-5937, a selective P2X3 antagonist, in patient subgroups defined by baseline awake cough frequencies. Am J Respir Crit Care Med. 2021;203:A1019.
- Birring SS, Morice AH, Smith J, et al. Baseline characteristics and burden of disease in populations defined by cough frequency tiers in RELIEF, a phase 2 study on the efficacy and safety of BLU-5937 in refractory chronic cough. Am J Respir Crit Care Med. 2021;203:A2357.
- Bellus Health announces positive topline results from its phase 2b SOOTHE trial of BLU-5937 for the treatment of refractory chronic cough. News release. Bellus Health; December 13, 2021. Accessed August 16, 2022. ir.bellushealth.com/news-releases/news-release-details/bellus-health-announces-positive-topline-results-its-phase-2b
- Bayer’s eliapixant significantly decreased cough frequency in phase IIb trial in patients with refractory chronic cough. News release. Evotec, Bayer; September 6, 2021. Accessed August 16, 2022. www.cmocro.com/news_detail/Bayer__039_s_Eliapixant_Significantly_Decreased_Cough_Freque/619034/index.html
- McGarvey L, Morice AH, Smith J, et al. Efficacy and safety of eliapixant in refractory chronic cough: results of the PAGANINI 12-week, randomized, placebo-controlled phase 2b study. Eur Resp J. 2021;58:PA562. doi:10.1183/13993003.congress-2021.PA562
- Bayer will discontinue phase II development candidate eliapixant. News release. Bayer HealthCare; February 4, 2022. Accessed August 15, 2022. media.bayer.com/baynews/baynews.nsf/id/8D5D4D684755D4C5C12587DF003C4A54?open&ref=irrefndcd
- Niimi A, Ishihara H, Hida A, Miyazaki S. Phase 2a randomized, double-blind, placebo-controlled, crossover study of a P2x3 receptor antagonist S-600918: effects on health-related quality of life in patients with refractory chronic cough. Am J Resp Crit Care Med. 2020;2(1):A7647.
- Smith J, Allman D, Badri H, et al. The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1). Chest. 2020;157(1):111-118. doi:10.1016/j.chest.2019.08.001
- Efficacy and Safety Study of Orvepitant for Chronic Cough in Patients with Idiopathic Pulmonary Fibrosis (IPF-COMFORT). Clinicaltrials.gov identifier: NCT05185089. Updated March 31, 2022. Accessed August 16, 2022. clinicaltrials.gov/ct2/show/NCT05185089
- Brozmanova M, Pavelkova N. The prospect for potent sodium voltage-gated channel blockers to relieve an excessive cough. Physiol Res. 2020;69(suppl 1):S7-S18. doi:10.33549/physiolres.934395
- Opioids. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; November 24, 2020. Updated June 2, 2022. www.ncbi.nlm.nih.gov/books/NBK547852/
- Trevi Therapeutics reports statistically significant result on interim analysis from the Ph2 CANAL Trial of nalbuphine ER in the treatment of chronic cough in idiopathic pulmonary fibrosis. News release. Trevi Therapeutics; February 24, 2022. Accessed August 15, 2022. apnews.com/press-release/pr-newswire/coronavirus-pandemic-business-health-new-haven-1d0fdc2c3195fe34cd11d5d953c5405a
- Nubain. Prescribing information. Par Pharmaceutical; 2016. Accessed August 16, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2016/018024s041lbl.pdf
