- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Suprachoroidal Triamcinolone Acetonide Injectable Suspension for Macular Edema Associated With Noninfectious Uveitis: An In-Depth Look at Efficacy and Safety
ABSTRACT
Patients with macular edema (ME) associated with uveitis (UME) are at risk for vision loss and decreased quality of life, and they often experience high health care costs and rates of workforce absenteeism. Systemically or locally delivered corticosteroids are the mainstay of treatment for UME. Although traditional corticosteroid treatments may demonstrate high levels of efficacy, systemic delivery carries the risk of potentially serious systemic adverse effects (AEs), and standard local modes of delivery may be associated with low bioavailability in posterior ocular tissues and steroid-associated AEs due to anterior ocular tissue exposure. Drug injection into the suprachoroidal space (SCS) allows for targeted delivery to chorioretinal tissues with high bioavailability in the posterior segment, as well as for inherent drug sequestration away from the anterior segment, which may lower the risk of AEs associated with anterior tissue exposure to steroids. A novel triamcinolone acetonide (TA) injectable suspension formulated for administration to the SCS, SCS-TA (Xipere®; Bausch + Lomb), received FDA approval in 2021 for the treatment of UME. It is administered via the SCS Microinjector® (Clearside Biomedical, Inc), a device specifically designed for SCS delivery of ocular therapeutics. This approval was based on results from the phase 3 PEACHTREE clinical trial (NCT02595398) that demonstrated the clinical efficacy—including significantly increased visual acuity and decreased central subfield thickness—and safety of SCS-TA in patients with UME. Results from this trial, as well as from its long-term observational extension (MAGNOLIA; NCT02952001) and an open-label safety study (AZALEA; NCT03097315), support the possibility that treatment with SCS-TA may address the burden of disease in patients with UME.
Am J Manag Care. 2023;29(suppl 2):S19-S28. https://doi.org/10.37765/ajmc.2023.89324
For author information and disclosures, see end of text.
Introduction
Uveitis describes ocular inflammation affecting any part of the uveal tract (ie, iris, ciliary body, and choroid) as a result of infectious or noninfectious (eg, autoimmune) processes (Figure 1).1 Uveitis is subclassified by the primary site of inflammation: Anterior uveitis primarily affects the anterior chamber tissues, intermediate uveitis primarily affects the vitreous, and posterior uveitis affects the retina or choroid.2,3 Panuveitis involves inflammation affecting all 3 sites, and pars planitis is a subclass of intermediate uveitis, which may also involve the peripheral retina.2,4 In 2015, uveitis was estimated to affect almost 330,000 adults in the United States, including approximately 300,000 cases of noninfectious uveitis (NIU).5
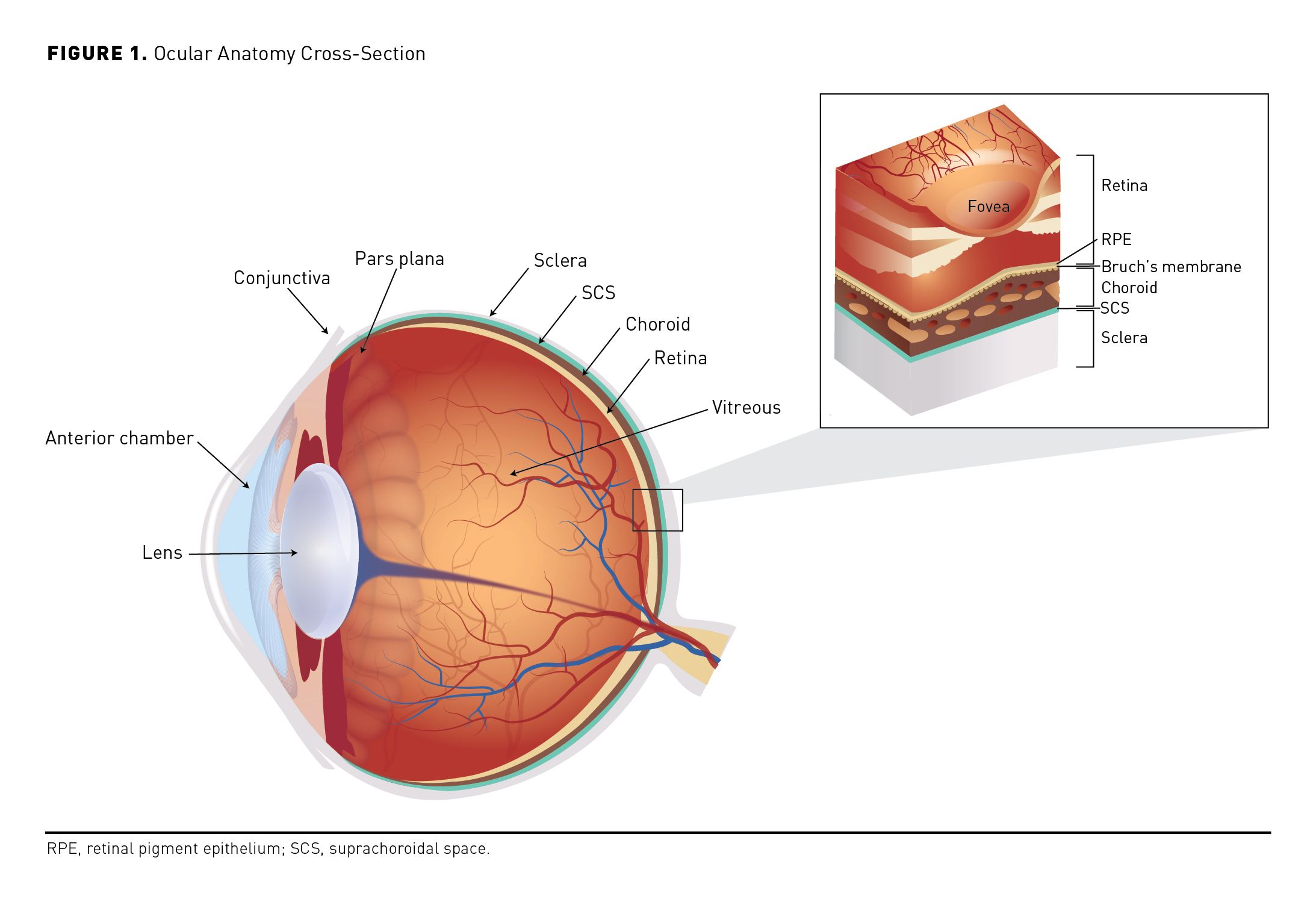
Macular edema (ME), a consequence of chronic uveitic inflammation, arises from an accumulation of extracellular fluid in intraretinal or subretinal spaces.2,6 It can result in retinal thickening and vision loss (often measured via central subfield thickness [CST] on ocular coherence tomography and best corrected visual acuity [BCVA] via Early Treatment Diabetic Retinopathy Study [ETDRS] letters, respectively).6,7 ME is the leading cause of vision loss in individuals with uveitis and it is the most common complication associated with NIU.2,6 The prevalence of ME varies widely based on the primary location of inflammation and there are limited data on the prevalence of ME in NIU.2 ME associated with uveitis (UME) may be observed in 9% to 28% of patients with anterior uveitis, 25% to 70% of those with intermediate uveitis, 19% to 34% of those with posterior uveitis, and 18% to 66% of those with panuveitis.2
This article summarizes the burden of disease on patients and payers, standard therapies and unmet needs in the treatment of UME, and the utility of the suprachoroidal space (SCS) as a target for drug delivery. It also presents data from clinical trials supporting the efficacy and safety of treatment with SCS-TA (Xipere®; Bausch + Lomb), a novel triamcinolone acetonide (TA) injectable suspension formulated for suprachoroidal delivery via the SCS Microinjector® (Clearside Biomedical, Inc) that received FDA approval for UME treatment in 2021.8,9
Clinical and Economic Burden of Disease
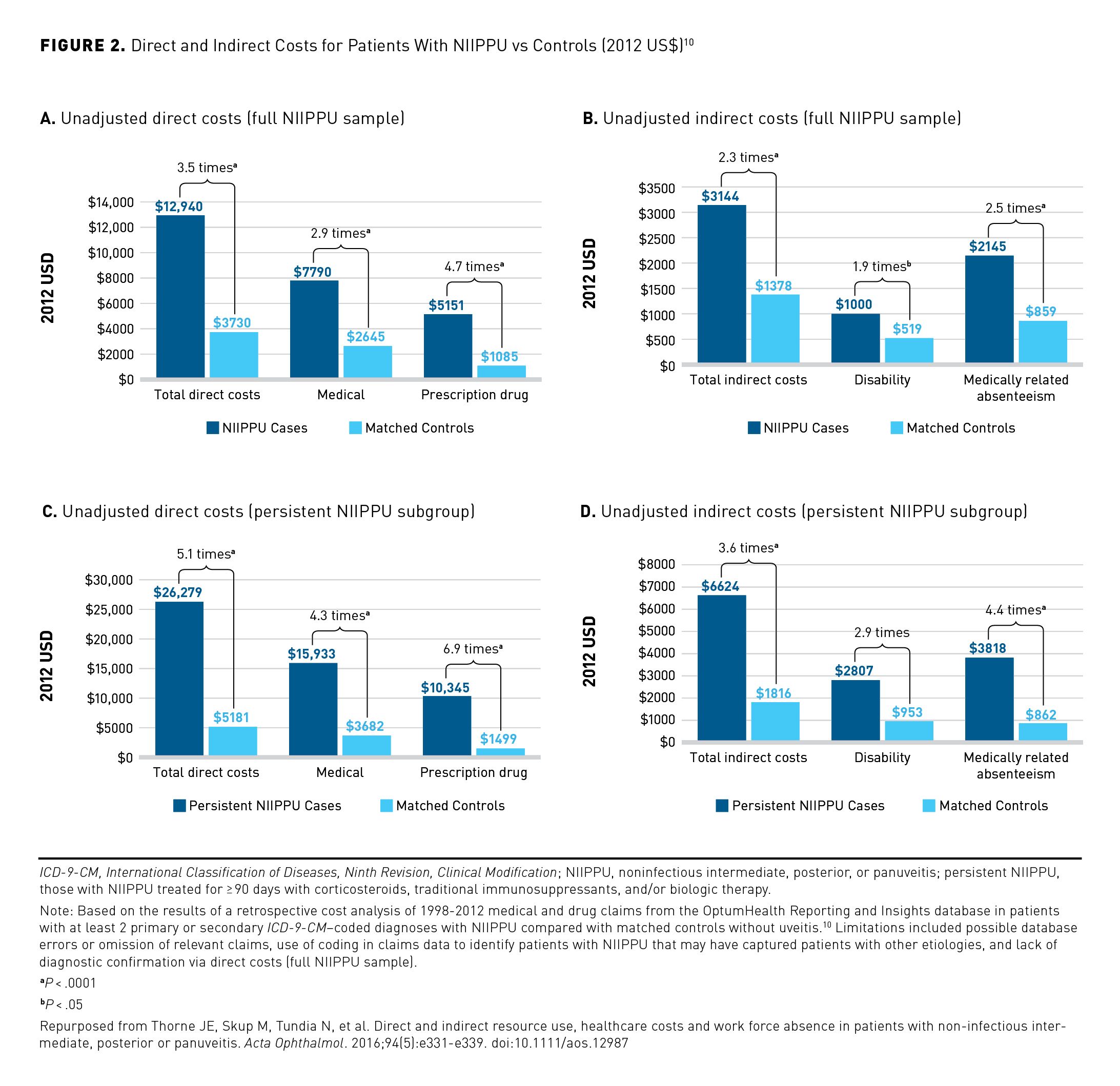
The disease burden in NIU and UME may include high rates of absenteeism and decreased quality of life (QOL) for patients, as well as substantial cost burdens on the US health care system. As NIU often affects patients during their most productive working years, increased absenteeism in those with posterior NIU and in those with persistent disease highlight the socioeconomic consequences of this disease and the importance of seeking improved treatments to alleviate this burden.10 From a payer perspective, these patient populations incur greater health care utilization (ie, inpatient, emergency department, outpatient/other visits, and prescription drug claims) and medical costs.10-12 Compared with matched controls without uveitis, a commercially insured population with NIU had significantly greater direct and indirect costs (Figure 2).10 In a separate study, total mean annual all-cause health care costs (2020 US$) were higher in a NIU population with UME compared with those with NIU alone ($19,851 vs $16,188); the differences were largely driven by greater outpatient and pharmacy costs.12
As UME is the leading cause of vision loss in NIU, there is a need for effective treatment that can increase visual acuity (VA) in patients with UME.2,12 UME is associated with higher rates of vision loss compared with NIU alone (5.7% vs 2.2%) and the rates of vision loss may be higher among those with UME who require treatment with a local steroid injection (7.9%).12 Evidence from the MUST (NCT00132691) randomized clinical trial (RCT) trial showed that visual impairment can negatively impact QOL in patients with NIU including those with UME, as a gain in BCVA of 5 ETDRS letters was associated with a significant improvement in QOL (P < .001).11,13 Thus, VA improvement is an outcome of key importance in UME treatment.2
Unmet Needs Associated with Standard UME Therapy
Corticosteroid therapy is a mainstay of treatment for UME and it is often administered as a primary treatment via systemic or local routes.2,14 Traditional corticosteroid delivery methods are often effective for treating UME, but they are associated with the risk of systemic and ocular AEs that may limit their clinical utility.1,3,14,15 In addition, certain methods of local drug delivery may be associated with low drug availability in posterior ocular tissues.1,3,14-17
Systemic and Topical Delivery
Systemic corticosteroid therapy, often recommended for the treatment of persistent or bilateral UME, is limited by the risk of systemic AEs, such as diabetes, osteoporosis, hypertension, gastrointestinal disturbance, and poor wound healing, as well as detrimental effects on mood and appetite, particularly with long-term use.2,3,14,15 Local treatment with topical corticosteroid drops may be preferred for treatment of mild or anterior disease, although this treatment can be associated with elevations in intraocular pressure (IOP) and formation of cataracts.14,15 In addition, efficacy of topical treatments may be limited in intermediate and posterior disease due to poor bioavailability in posterior tissues; thus, topical treatment requires frequent application, with the concomitant potential for low patient adherence.1,15,17
Periocular and IVT Injections
Local corticosteroid delivery to the posterior segment can be achieved by delivering the drug adjacent to the sclera through periocular injection or directly into the vitreous of the eye through intravitreal (IVT) injection or implantation.16 Periocular and IVT corticosteroid injections are recommended as effective treatment for first-line treatment of unilateral UME.14,16 IVT injection is the standard mode of treatment delivery for disease affecting the posterior segment.16,17 In periocular injection, the drug is delivered as a depot outside of the eye and it must pass through several anatomical barriers to reach the choroid, retinal pigment epithelium (RPE), or retina. Therefore, this method may result in low or inconsistent drug delivery and resultant low bioavailability in the posterior segment.1,16 The most common AEs associated with periocular or IVT injection of corticosteroids include elevated IOP and development of cataracts.14,15,17,18 For these procedures, some rare but serious procedure-related complications include vitreous hemorrhage, retinal tear and detachment, and endophthalmitis.14-18
IVT Steroid Implants
IVT steroid implants are indicated for NIU affecting the posterior segment of the eye and can improve UME through the sustained release of corticosteroids (typically, over the course of 6-36 months).14,15,19-22 IVT steroid implants may be used as adjunctive treatment with systemic steroids or immunosuppression for bilateral disease, or as monotherapy for persistent or recurrent unilateral disease,2,14 particularly in patients with periodic disease exacerbations that punctuate periods of inactivity. AEs associated with IVT steroid implants may include endophthalmitis, eye pain or inflammation, conjunctival hemorrhage, and retina or vitreous detachment; also possible are cataract formation and elevated IOP associated with anterior ocular tissue exposure to steroids over time.1,19-21
Comparative Efficacy of Corticosteroid Delivery Methods
The POINT (NCT02374060) and MUST RCTs compared outcomes of various corticosteroid treatments in patients with NIU with or without ME (Table 111,18,20,21,23-25).23,25 In POINT, IVT injection and IVT implant were demonstrated to be superior to periocular injection for treating UME with respect to improvement in both BCVA and CST.23
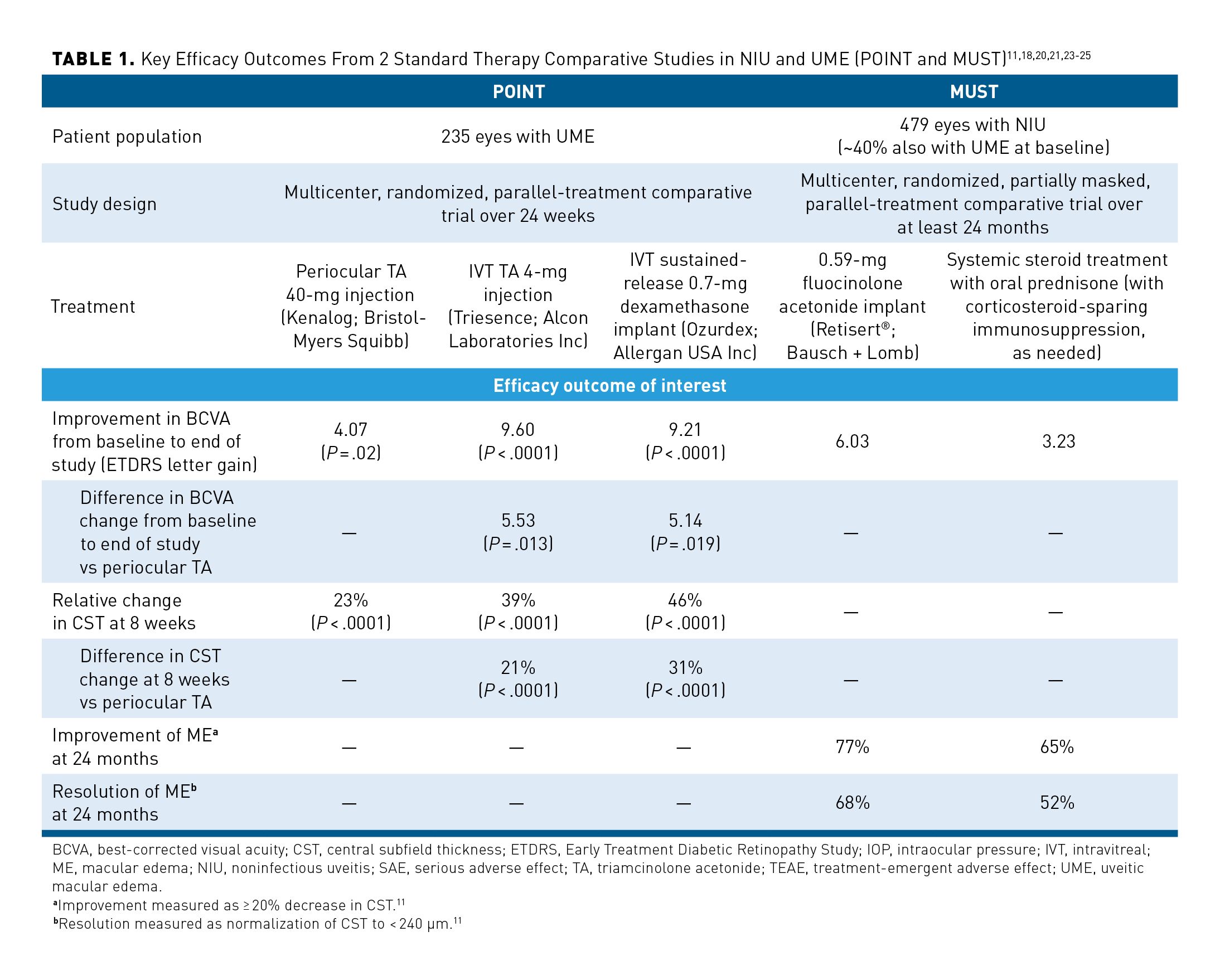
In the MUST trial, among patients with ME, nearly one-third of patients treated with either IVT implant or systemic corticosteroid therapy did not achieve improvement in their ME, and after 2 years of follow-up, ME remained unresolved in 40% of eyes.11
There are ongoing unmet needs for safe, effective treatments specific to UME that result in high drug bioavailability in posterior tissues and carry a lower risk of AEs associated with anterior tissue exposure. Innovative treatment approaches may meet such needs, particularly if they carry the potential for improved efficacy with a concomitant reduction in corticosteroid-associated AEs.
Injection Into the Suprachoroidal Space
Injection into the SCS offers a unique alternative mode of delivery that may minimize AEs inherent to local ocular steroid delivery.26 The SCS is a potential space between the choroid and the sclera that expands upon injection of fluid, providing an innovative delivery target for ocular therapeutics.17,26
After injection into the SCS, fluid flows posteriorly, allowing for distribution of therapeutic agents to posterior tissues and a high degree of bioavailability in the adjacent choroid, RPE, and retina.26,27 Furthermore, injection into the SCS has been shown to limit drug exposure to the vitreous humor and to anterior segment tissues, including the lens, aqueous humor, iris/ciliary body, and cornea.1,17,26,28 Thus, SCS injection of corticosteroids is likely to carry a lower risk of AEs associated with anterior tissue exposure to steroids.1,17
Until recently, drug delivery to the SCS has been limited in use and has required an operating room setting with immobilization of the patient’s eye under anesthesia and surgical dissection through the sclera.26 However, reliable outpatient-based injection into the SCS can now be achieved with use of a microneedle.26 The SCS Microinjector® was designed for SCS microneedle delivery of ocular therapeutics such as TA.
Pharmacokinetics in the SCS
An investigational prototype of a TA injectable suspension (X-TA) was formulated for use in the SCS and optimized for injection via SCS Microinjector®.28 X-TA formulation modifications sought to ensure consistency of drug performance and injectability of the agent.28 The glide force (the necessary pressure applied to the plunger to expel a formulation from the injector) of 2 TA formulations—TRI (Triesence; Alcon Laboratories Inc; FDA approved for uveitis and several other ocular diseases)18 and X-TA—were assessed with the SCS Microinjector®.28 TRI injection showed significantly higher average glide force than did X-TA injection (P < .001), and X-TA had a much lower rate of variability in glide force over time.28 The improved glide force characteristics of X-TA are expected to provide greater tactile perception for the injecting clinician, who relies on feeling a loss of resistance to detect when the microneedle tip reaches the SCS.28 Thus, clinicians performing injections of TA formulations optimized for microinjection may achieve more controlled and consistent delivery into the SCS with the SCS Microinjector®.28
Results from preclinical studies support the utility of TA delivery into the SCS via SCS Microinjector® to achieve compartmentalized, durable corticosteroid delivery to the posterior segment of the eye.28 In one animal study, compared with IVT injection, SCS injection was associated with greater sequestration of TA away from anterior tissues, as well as higher levels of TRI in the RPE, choroid, and sclera over the course of 3 months.28 These results demonstrate that SCS injection can result in high bioavailability and durability of TA in posterior tissues and potentially support a lower risk of IOP elevation and cataract development associated with corticosteroid exposure to anterior tissues.28
Clinical Evidence Supporting Use of SCS-TA
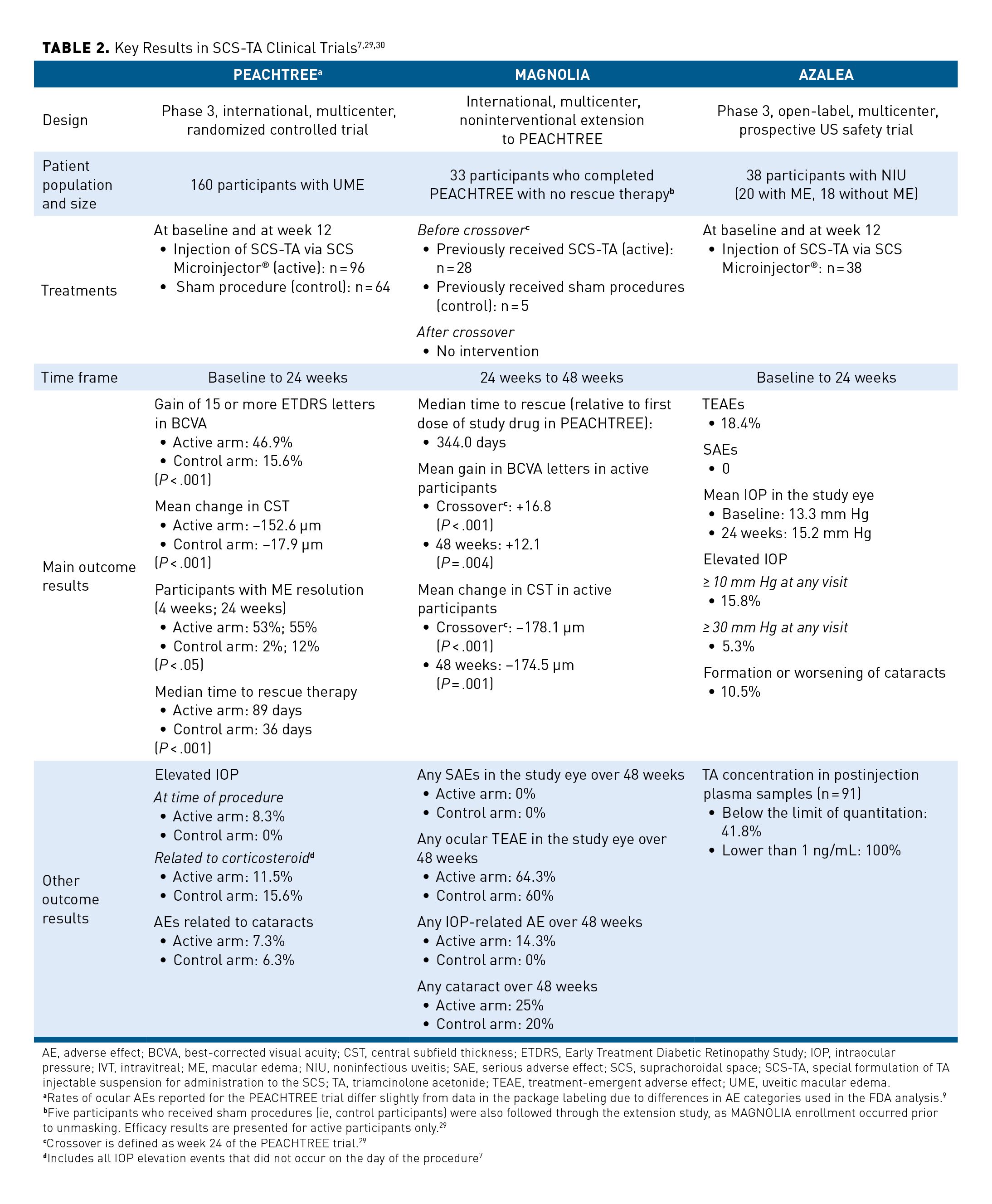
The safety and efficacy of SCS-TA delivered via SCS Microinjector® for the treatment of UME was assessed across 3 clinical trials: PEACHTREE (NCT02595398), MAGNOLIA (NCT02952001), and AZALEA (NCT03097315) (Table 2).7,29,30
PEACHTREE Pivotal Trial
Efficacy and safety results from the 24-week, international, multicenter, double-masked PEACHTREE phase 3 pivotal RCT led to FDA approval of SCS-TA for the treatment of UME.7,8 A total of 160 adult participants with ME associated with NIU were randomized (3:2) to receive either an SCS-TA microinjection (active; n = 96) or a sham procedure mimicking SCS microinjection (control; n = 64) in 1 eye at day 0 and week 12, with assessments performed every 4 weeks up to 24 weeks (Table 27,29,30).7
Results showed that significantly more participants in the SCS-TA arm achieved the primary end point of gaining at least 15 ETDRS letters in BCVA at week 24 than did those in the control arm (46.9% vs 15.6%; P < .001) (Figure 3A).7 By week 8, significantly more participants in the SCS-TA arm achieved vision of at least 70 EDTRS letters compared with the control arm (49% vs 15%, respectively; P < .001).7 Differences in these outcomes were significant in favor of SCS-TA at all time points (Figure 3B and Figure 3C).7 The secondary end point of mean change in CST at week 24 was greater in the SCS-TA arm than in the control arm (P < .001).7 In addition, significantly more participants in the SCS-TA arm showed ME resolution (defined as CST < 300 µm) than did participants in the control arm at all visits through week 24 (P < .05).7
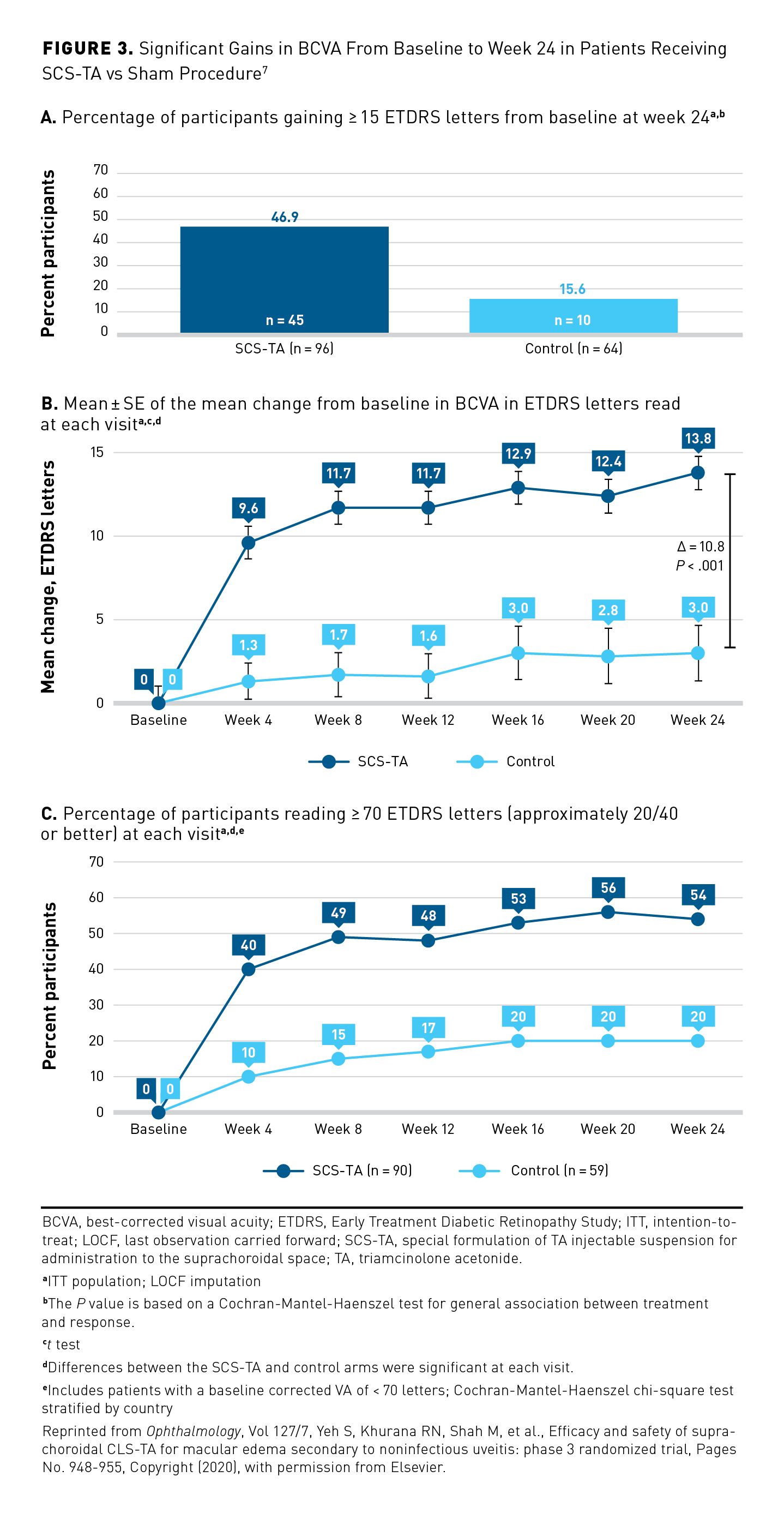
In the safety analysis, participants treated with SCS-TA experienced a higher rate of IOP AEs at the time of the procedure (due to the expected volume effect), but a lower rate of IOP elevation occurring after the day of the procedure), than did the participants in the control arm, while rates of AEs related to cataracts were similar between the arms.7 All IOP-related AEs in the control arm occurred after receipt of rescue therapy with local corticosteroids (ie, IVT or periocular corticosteroid injection).7
Post Hoc Analyses of PEACHTREE
The PEACHTREE trial did not compare SCS injection of SCS-TA with other available NIU treatments.7 In clinical practice, treatment of uveitis and of UME may require a combination of systemic and local therapies.2,14 Two separate post hoc analyses of PEACHTREE explored rescue medication use and the combination of systemic and local therapies.
A post hoc analysis of the PEACHTREE trial compared efficacy and safety data from the 83 participants receiving SCS-TA who did not receive rescue medication during the study (ie, unrescued) with data from 46 participants in the sham control arm who required rescue medication (ie, rescued) (Table 331,32).31 Among rescued patients in the control arm, 80.4% received IVT or periocular corticosteroids.31 At week 24, there was a trend of greater VA gains in the unrescued SCS-TA arm than in the rescued control arm, although differences between groups were nonsignificant both in the proportion of participants gaining at least 15 BCVA letters (P = .115) and in the mean letter gain (P = .08).31 However, unrescued SCS-TA participants did experience a significantly greater mean reduction in CST than did rescued control participants (P = .04).31
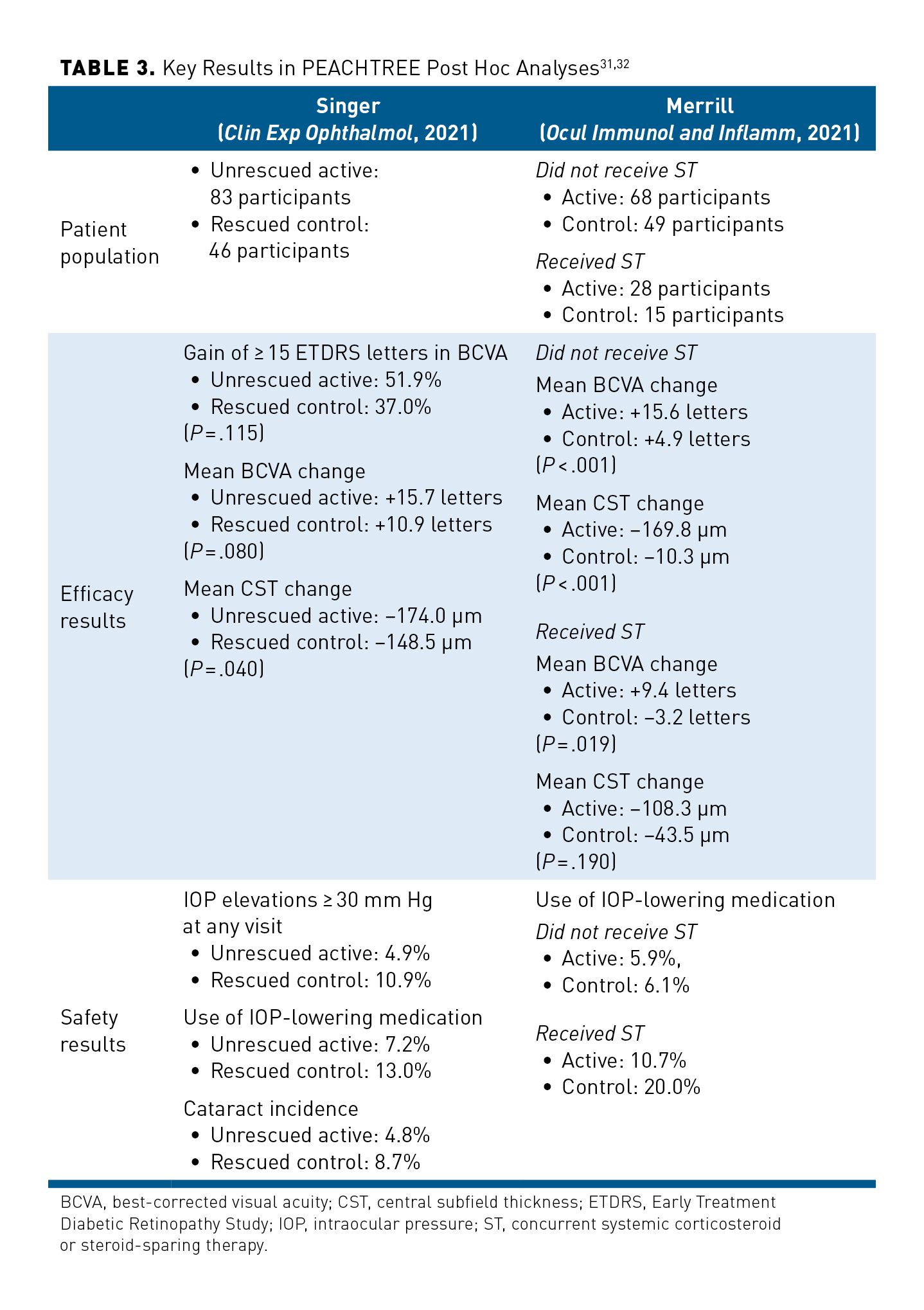
Incidence of AEs involving IOP appeared lower in the unrescued SCS-TA arm than in the rescued control arm.31 AEs related to elevated IOP occurred in 10.8% of unrescued SCS-TA participants and in 21.7% of the rescued control patients.31 Rescued control participants had nearly twice the incidence of cataracts (8.7% vs 4.8%) and use of IOP-lowering medication (13.0% vs 7.2%), and more than twice as many control participants experienced an IOP of at least 30 mm Hg at any visit (10.9% vs 4.9%), compared with unrescued SCS-TA participants, respectively.31 One important limitation of this study is that unrescued SCS-TA participants had longer exposure to corticosteroids than did rescued control patients, which may have influenced efficacy outcomes in favor of unrescued SCS-TA participants and safety outcomes in favor of rescued control participants.31
A second post hoc analysis of the PEACHTREE trial examined the effect of baseline corticosteroid and/or steroid-sparing systemic treatment (ST) use on study outcomes (Table 331,32).32 Among participants with no concurrent baseline ST use, those in the SCS-TA arm had a significantly higher gain in mean BCVA and greater reduction in CST compared with those in the control arm (both P < .001) at week 24.32 Among participants who received concurrent ST at baseline, those given SCS-TA also had a significantly greater mean change in BCVA compared with those in the control arm (P = .019) at week 24 as well as a trend in greater reduction in CST.32
Rates of IOP-lowering–medication use were similar between the SCS-TA and control arms that did not receive ST; however, among participants who did receive ST, medication use was approximately doubled in the control arm compared with the SCS-TA arm.32 While results were limited by the small sample size in each subgroup, efficacy and safety results were generally consistent with the findings in PEACHTREE, supporting the clinically meaningful benefit of SCS-TA in patients with UME, regardless of concurrent systemic therapy use.32
MAGNOLIA Extension Study
The MAGNOLIA trial was an international, multicenter, noninterventional, 24-week extension study of PEACHTREE in 33 participants (SCS-TA arm, 28 participants; control arm, 5 participants) who did not require rescue medications over the course of the original study.29 The objective of this study was to assess durability of SCS-TA efficacy, with a primary end point of time to rescue from the first treatment in PEACHTREE.29 Follow-up visits were conducted every 6 weeks through 24 weeks (ie, 48 weeks from PEACHTREE baseline).29
The results of the MAGNOLIA trial provided further longitudinal outcomes from the PEACHTREE study, indicating that the efficacy and safety of SCS-TA were maintained in participants for up to 9 months following the 12-week treatment in PEACHTREE (Table 27,29,30).29 Unrescued participants who received SCS-TA in PEACHTREE showed a significant mean BCVA gain and mean reduction in CST between PEACHTREE baseline and crossover (ie, MAGNOLIA day 0; both P < .001), and both end point results remained significant at week 48 (ie, MAGNOLIA week 24; P = .004 and P = .001, respectively).29 A Kaplan-Meier analysis conducted post hoc evaluated the risk of rescue therapy over the entire 48-week combined study period and included all PEACHTREE study participants.29 Results showed that the median time from last dose of study drug to need for rescue medication was longer in the SCS-TA arm than in the control arm by more than 200 days (257.0 days vs 55.5 days, respectively), and the time-to-rescue distributions were significantly different (P < .001).29 Because no study drug was administered in the MAGNOLIA trial, these results—showing maintenance of significant improvements in BCVA and CST and an extended time to rescue medication in the SCS-TA arm—demonstrate the long-term efficacy and duration of action seen with SCS-TA over 48 weeks.29
Safety results in MAGNOLIA were generally consistent with those seen in PEACHTREE, with no observed serious AEs (SAEs) in the study eye in either group over the course of 48 weeks.29 The MAGNOLIA trial provides additional information related to the efficacy, safety, and potential treatment durability following SCS-TA injection.7,29
AZALEA Safety Trial
The AZALEA study was an open-label, prospective, multicenter US safety trial that evaluated safety of SCS-TA administered via the SCS Microinjector® as 1 unilateral injection on day 0 and at week 12 among 38 adult participants with NIU with (n = 20) or without (n = 18) ME, with follow-up every 4 weeks for 24 weeks (Table 27,29,30).30 The primary outcome was incidence of treatment-emergent AEs (TEAEs) and SAEs after 24 weeks.30 Over the course of the study, 7 (18.4%) of the participants experienced a TEAE deemed by an investigator to be treatment-related. No TEAEs led to study discontinuation and there were no SAEs involving the study eye.30
Mean IOP in the study eye was 13.3 mm Hg at baseline and 15.2 mm Hg at week 24.30 Compared with baseline, IOP elevations of more than 10 mm Hg occurred in 6 participants (15.8%), and IOP elevations of 30 mm Hg or more occurred in 2 participants (5.3%).30 One event of elevated IOP occurred at time of procedure.30 In addition, cataract formation or worsening occurred in 4 participants (10.5%), with 1 case considered related to treatment; no participants required cataract surgery.30 The concentration of TA in plasma samples collected at various time points throughout the study was below 1 ng/mL or undetectable in all samples.30 These results further support the safety of SCS-TA administered via SCS injection in patients with UME.
Discussion
Results from the PEACHTREE, MAGNOLIA, and AZALEA clinical trials demonstrate the increases in VA, decreases in CST, and favorable ocular safety seen with SCS-TA treatment delivered via SCS Microinjector®. Data from the PEACHTREE trial demonstrated the treatment efficacy of SCS-TA administered via SCS Microinjector® in participants with UME, with nearly half the participants gaining at least 15 ETDRS letters (3-line improvement), which represents a doubling of the visual angle; the clinical significance of these results could mean the difference between 20/80 vision (moderate visual impairment) and 20/40 vision (the vision standard for unrestricted legal driving in the United States).7,33,34 Indeed, by week 8 of PEACHTREE, nearly half of participants who were treated with SCS-TA were able to read at least 70 EDTRS letters (ie, 20/40 Snellen equivalent) vs 15% of participants in the control arm, a difference that remained significant through the end of the 24-week study.7 Additionally, the VA efficacy results seen in MAGNOLIA reinforce SCS-TA’s duration of action in patients with UME, showing a sustained benefit over many months.29 Results from this clinical program indicate the potential of SCS-TA to substantially improve QOL for patients living with UME-associated vision loss.
An animal model study first revealed the drug sequestration to the posterior segment possible with TA injection into the SCS, indicating the potential for reduced AEs associated with corticosteroid treatment compared with other delivery modalities.28 In the POINT study, more than 30% of eyes receiving IVT TRI, IVT dexamethasone implant, or periocular TA were treated with IOP-lowering medications by the end of the 24 weeks.23 Although between-trial comparisons have limitations, only 7.3% of PEACHTREE participants treated with SCS-TA required use of IOP-lowering medications, suggesting that this treatment may indeed be associated with IOP-sparing benefits vs standard delivery methods.7 SCS injection of SCS-TA has been shown to be effective and with a favorable safety profile compared with the standard of care, representing a beneficial therapeutic innovation that may address ongoing unmet needs in the treatment of UME.
To inform payer reimbursement decisions in the UME treatment landscape, a simulated US adult patient-level model estimated the cost-effectiveness of SCS-TA vs best supportive care from a payer perspective over a 10-year horizon using patient characteristics and clinical outcomes derived from PEACHTREE.35 Results showed that SCS-TA was likely cost-effective vs best supportive care at willingness-to-pay thresholds of $50,000 or more (2020 US$) per quality-adjusted life-year gained.35 SCS-TA is the first therapy approved by the FDA specifically for the treatment of UME, and there are currently no clinical US guidelines for treatment of this disease.35 Treatments that can reduce UME and related vision loss may improve patient QOL and lead to lower rates of health care utilization and absenteeism.10,13,35 Thus, evidence supporting the clinical efficacy and safety as well as the cost-effectiveness of this treatment serve to inform both clinician treatment choices and payer reimbursement decision-making.35
Conclusions
Standard methods of corticosteroid delivery, while often beneficial in reducing ME and improving VA, confer the risk of AEs when administered systemically or locally, including ocular hypertension, glaucoma, and cataract resulting from steroid exposure in anterior tissues. In addition, certain traditional delivery methods may result in poor drug bioavailability in posterior tissues. Administration of corticosteroids into the SCS offers an alternative solution for many challenges associated with current UME therapy. SCS-TA is the first drug to receive FDA approval for delivery into the SCS and is the first drug to be approved for UME treatment. TA injection into the SCS via SCS Microinjector® is associated with high drug bioavailability in posterior tissues and sequestration away from unaffected anterior tissues. Based on the results of the PEACHTREE, MAGNOLIA, and AZALEA clinical trials, UME treatment with SCS-TA is associated with high therapeutic efficacy and a decreased risk of steroid-associated ocular AEs, presenting an opportunity to reduce the burden of disease for patients and health care payers.
For more information about the utility of the SCS, click here.
Authorship affiliation: University of Nebraska Medical Center (SY); Clearside Biomedical (TC); Indiana University School of Medicine (TC)
Funding source: This work is supported by Bausch + Lomb.
Author disclosure: Dr Yeh reports serving on a consultancy or paid advisory board for Bausch + Lomb, Adverum Biotechnologies, and REGENXBIO. Dr Ciulla is employed by and owns stock in Clearside Biomedical, which developed Xipere.
Authorship information: Concept and design (SY, TC); acquisition of data (SY); analysis and interpretation of data (SY, TC); drafting of the manuscript (SY); critical revision of the manuscript for important intellectual content (SY, TC); administrative, technical, or logistic support (SY); supervision (SY, TC).
Address correspondence to: Steven Yeh, MD. Truhlsen Eye Institute, 3902 Leavenworth Street. Omaha, NE 68105. Email: syeh@unmc.edu
REFERENCES
- Valdes LM, Sobrin L. Uveitis therapy: the corticosteroid options. Drugs. 2020;80(8):765-773. doi:10.1007/s40265-020-01314-y
- Massa H, Pipis SY, Adewoyin T, Vergados A, Patra S, Panos GD. Macular edema associated with non-infectious uveitis: pathophysiology, etiology, prevalence, impact and management challenges. Clin Ophthalmol. 2019;13:1761-1777. doi:10.2147/OPTH.S180580
- Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol. 2014;8:1891-1911. doi:10.2147/OPTH.S47778
- Pars planitis. National Organization for Rare Disorders. Updated 2008. Accessed January 12, 2023. https://rarediseases.org/rare-diseases/pars-planitis/
- Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237-1245. doi:10.1001/jamaophthalmol.2016.3229
- Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. Eye (Lond). 2016;30(10):1277-1292. doi:10.1038/eye.2016.115
- Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948-955. doi:10.1016/j.ophtha.2020.01.006
- Bausch’s and Clearside’s eye injection secures FDA approval. News release. FDA; October 26, 2021. Accessed January 12, 2023. https://www.fdanews.com/articles/205018-bauschs-and-clearsides-eye-injection-secures-fda-approval
- Xipere. Prescribing information. Bausch + Lomb; 2022. Accessed January 12, 2023. https://pi.bausch.com/globalassets/pdf/packageinserts/vision-care/xipere_prescribing_information.pdf
- Thorne JE, Skup M, Tundia N, et al. Direct and indirect resource use, healthcare costs and work force absence in patients with non-infectious intermediate, posterior or panuveitis. Acta Ophthalmol. 2016;94(5):e331-e339. doi:10.1111/aos.12987
- Tomkins-Netzer O, Lightman S, Drye L, et al; Multicenter Uveitis Steroid Treatment Trial Research Group. Outcome of treatment of uveitic macular edema: the Multicenter Uveitis Steroid Treatment trial 2-year results. Ophthalmology. 2015;122(11):2351-2359. doi:10.1016/j.ophtha.2015.07.036
- Hariprasad SM, Joseph G, Gagnon-Sanschagrin P, et al. Healthcare costs among patients with macular oedema associated with non-infectious uveitis: a US commercial payer’s perspective. BMJ Open Ophthalmol. 2021;6(1):e000896. doi:10.1136/bmjophth-2021-000896
- Frick KD, Drye LT, Kempen JH, et al; Multicenter Uveitis Steroid Treatment–MUST Trial Research Group. Associations among visual acuity and vision- and health-related quality of life among patients in the Multicenter Uveitis Steroid Treatment Trial. Invest Ophthalmol Vis Sci. 2012;53(3):1169-1176. doi:10.1167/iovs.11-8259
- Koronis S, Stavrakas P, Balidis M, Kozeis N, Tranos PG. Update in treatment of uveitic macular edema. Drug Des Devel Ther. 2019;13:667-680. doi:10.2147/DDDT.S166092
- Conrady CD, Yeh S. A review of ocular drug delivery platforms and drugs for infectious and noninfectious uveitis: the past, present, and future. Pharmaceutics. 2021;13(8):1224. doi:10.3390/pharmaceutics13081224
- Varela-Fernández R, Díaz-Tomé V, Luaces-Rodríguez A, et al. Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12(3):269. doi:10.3390/pharmaceutics12030269
- Wan C-R, Muya L, Kansara V, Ciulla TA. Suprachoroidal delivery of small molecules, nanoparticles, gene and cell therapies for ocular diseases. Pharmaceutics. 2021;13(2):288. doi:10.3390/pharmaceutics13020288
- Triesence. Prescribing information. Alcon Laboratories Inc; 2007. Accessed January 12, 2023.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/022223,022048lbl.pdf - Yutiq. Prescribing information. EyePoint Pharmaceuticals US Inc; 2022. Accessed January 12, 2023. https://yutiq.com/downloads/YUTIQ-US-PI-022022.pdf
- Retisert. Prescribing information. Bausch + Lomb; 2021. Accessed January 12, 2023. https://www.bauschretinarx.com/siteassets/retisert/pdf/retisert-prescribing-information.pdf
- Ozdurex. Prescribing information. Allergan USA Inc; 2022. Accessed January 12, 2023. https://www.rxabbvie.com/pdf/ozurdex_pi.pdf
- Chang-Lin J-E, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80-86. doi:10.1167/iovs.10-5285
- Thorne JE, Sugar EA, Holbrook JT, et al; Multicenter Uveitis Steroid Treatment Trial Research Group. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283-295. doi:10.1016/j.ophtha.2018.08.021
- Kenalog. Prescribing information. Bristol-Myers Squibb; 2019. Accessed January 12, 2023.
https://packageinserts.bms.com/pi/pi_kenalog-40-80.pdf - Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group; Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment trial. Ophthalmology. 2011;118(10):1916-1926. doi:10.1016/j.ophtha.2011.07.027. Published correction appears in Ophthalmology. 2012;119(2):212.
- Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:58-66. doi:10.1016/j.addr.2018.03.001
- Seiler GS, Salmon JH, Mantuo R, Feingold S, Dayton PA, Gilger BC. Effect and distribution of contrast medium after injection into the anterior suprachoroidal space in ex vivo eyes. Invest Ophthalmol Vis Sci. 2011;52(8):5730-5736. doi:10.1167/iovs.11-7525
- Muya L, Kansara V, Cavet ME, Ciulla T. Suprachoroidal injection of triamcinolone acetonide suspension: ocular pharmacokinetics and distribution in rabbits demonstrates high and durable levels in the chorioretina. J Ocul Pharmacol Ther. 2022;38(6):459-467. doi:10.1089/jop.2021.0090
- Khurana RN, Merrill P, Yeh S, et al. Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA). Br J Ophthalmol. 2022;106(8):1139-1144. doi:10.1136/bjophthalmol-2020-317560
- Henry CR, Shah M, Barakat MR, et al. Suprachoroidal CLS-TA for non-infectious uveitis: an open-label, safety trial (AZALEA). Br J Ophthalmol. 2022;106(6):802-806. doi:10.1136/bjophthalmol-2020-318019
- Singer MA, Merrill P, Yeh S, Hall C, Kapik B, Ciulla TA. Suprachoroidal triamcinolone acetonide versus rescue therapies for the treatment of uveitic macular oedema: a post hoc analysis of PEACHTREE. Clin Exp Ophthalmol. 2021;50(1):23-30. doi:10.1111/ceo.14024
- Merrill PT, Henry CR, Nguyen QD, Reddy A, Kapik B, Ciulla TA. Suprachoroidal CLS-TA with and without systemic corticosteroid and/or steroid-sparing therapy: a post-hoc analysis of the phase 3 PEACHTREE clinical trial. Ocul Immunol Inflamm. Published online August 18, 2021. doi:10.1080/09273948.2021.1954199
- Reddy V. Driving restrictions per state. American Academy of Ophthalmology EyeWiki. January 6, 2023. Accessed January 12, 2023. https://eyewiki.aao.org/Driving_Restrictions_per_State
- Whitcup SM. Clinical examination of the patient with uveitis. In: Whitcup SM and Sen HN, eds. Whitcup and Nussenblatt’s Uveitis—Fundamentals and Clinical Practice. 5th ed. Elsevier; 2020:31-41. doi:10.1016/B978-0-323-48014-7.00003-8. ScienceDirect. Accessed January 12, 2023. https://www.sciencedirect.com/science/article/pii/B9780323480147000038?via%3Dihub
- Bhattacharyya S, Hariprasad SM, Albini TA, et al. Suprachoroidal injection of triamcinolone acetonide injectable suspension for the treatment of macular edema associated with uveitis in the United States: a cost-effectiveness analysis. Value Health. 2022;25(10):1705-1716. doi:10.1016/j.jval.2022.07.008
