- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Starting Insulin, GLP-1 at Same Time Brings Better Glycemic Control, Real-World Data Show
Selected abstracts from the American Diabetes Association's 80th Scientific Sessions discuss when to add injectable therapy, how patients who switched to semaglutide lost more weight and gained glycemic control, and offered results from an early-phase study on a monoclonal antibody that may preserve B-cell function.
The question of when to add more glucose-lowering therapies—and in what order—has been one of the more important discussions in diabetes treatment in recent years. The 2020 guidelines from the American Diabetes Association (ADA) called for adding sodium glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists to treat patients with type 2 diabetes (T2D) who have comorbidities.
A year prior, the ADA had endorsed using a GLP-1 receptor agonist or a fixed-dose combination therapy before insulin if patients needed an injectable therapy achieve control.
Data presented this weekend at the ADA's 80th Scientific Sessions reinforced the idea that a combination therapy makes sense before moving to insulin alone—and for some patients, a combination may be the right choice from the start. Using claims data from the Optum Humedica database, from January 1, 2011, through June 30, 2017, research supported by Sanofi suggests that starting the 2 injectable drugs at roughly the same time produces the best glycemic control.
Sanofi, which makes Soliqua, a fixed-dose injectable therapy of insulin glargine and lixisenatide, announced the results in a press release, and senior author Vivian Fonseca, MD, assistant dean for clinical research at Tulane University School of Medicine, presented them in a virtual poster.1 Soliqua competes with Novo Nordisk’s Xultophy, a combination of insulin degludec and liraglutide.
According to Fonseca’s presentation, investigators screened more than 5.8 million patient records to evaluate those patients with T2D who met the following criteria:
- 18 to 80 years of age
- Glycated hemoglobin (A1C) ≥9% at baseline
- Started a GLP-1 receptor agonist or a basal insulin and then started a second prescription of the other drug class within 12 months
- All patients had to be treated with oral antidiabetic therapies before taking injectable therapy
- Patients had to have at least 1 additional A1C recorded after the index date.
Investigators reviewed data for 6339 patients and divided them into 5 groups based on the number of days between the 2 prescriptions: 30 days or less, 31 to 90 days, 91 to 180 days, 181 to 270 days, and 271 to 360 days.
Those who started insulin and a GLP-1 receptor agonist within 30 days of each other had the best glycemic control across several measures: At 6 months, they saw the highest percentage of patients achieve an A1C of <7% (24.4%), the highest achieve an A1C of <8% or less (45.3%), and they had the highest share of patients achieve A1C reductions of at least 2% (53.0%) and at least 1% (72.2%). The larger the gap between the 2 prescription starts, the less likely patients in the group were to achieve an A1C or 8% or less. Only about half the those who received the 2 prescriptions in the 31 to 90 day window achieved an A1C reduction of 2% (47.2% at 6 months, 49.2% at 12 months).
Once the gap between prescriptions reached 91 days, the ability to achieve control fell off significantly; between 33.3% and 36.7% achieved a 2% reduction at 6 months and 37.2% to 37.6% achieved this at 12 months. Less than one-third of these patients were able to achieve an A1C target of 8% at either the 6- or 12-month mark.
“People with severely uncontrolled diabetes may benefit from initiating both basal insulin and GLP-1 receptor agonists in close time proximity, for example, within 90 days, rather than waiting to assess the effectiveness of a single injectable therapy,” Fonseca said during his presentation.
While weight loss was about the same for both groups, patients saw a slight increase in weight at the 12-month mark if they added the second injectable between 181 and 270 days. One of the benefits of combination therapy is the ability of the GLP-1 receptor agonist to mitigate the tendency of insulin to cause patients to gain weight.
Sanofi’s Rogelio Braceras, MD, North America Medical Head of General Medicines, said in a statement that ADA guidelines call for combination therapy in patients who are 1.5% to 2% above A1C goals. “The complementary actions of a GLP-1 receptor agonist and an insulin, when initiated simultaneously, may provide an effective approach for adults with uncontrolled type 2 diabetes.”
The claims study did not include time-in-range data, which are of increased interest to clinicians and to patients, but a Sanofi spokesman said that several continuous glucose monitoring studies with Soliqua are under way.
TIGER: Golimumab Preserves B-Cell Function, Reduces Insulin Use in Children
For more than a decade, scientists have sought a solution for preserving remaining B-cell function in patients newly diagnosed with type 1 diabetes (T1D), before the attack that destroys a body’s ability to produce insulin wipes it out. TIGER evaluated golimumab, an IgG1κ monoclonal antibody specific to tumor necrosis factor α (TNFα) and examined whether this treatment could preserve B-cell function in children and young adults with newly diagnosed stage III T1D.
In this phase 2a, double-blind placebo-controlled study, 84 randomized participants aged 6 to 21 years were assigned to receive golimumab (56 patients) or placebo (28 patients). Both the study drug and the placebo were given subcutaneously.2 The primary end point was C-peptide area under the curve (AUC) at week 52 after a 4-hour mixed-meal tolerance test. Insulin use, A1C, hypoglycemia rates, and proinsulin/C-peptide ratios were measured.
The study was positive. Mean (SD) 4-hour C-peptide AUC was 0.64 (0.423) pmol/mL for the study drug and 0.43 (0.388) pmol/mL for placebo, P <.001. (Figure 1). Researchers reported that golimumab was well-tolerated and showed the ability to “preserve endogenous insulin production and improve clinical and metabolic parameters in children and young adults with newly diagnosed stage 3 T1D.”
FIGURE 1. TIGER Study Results
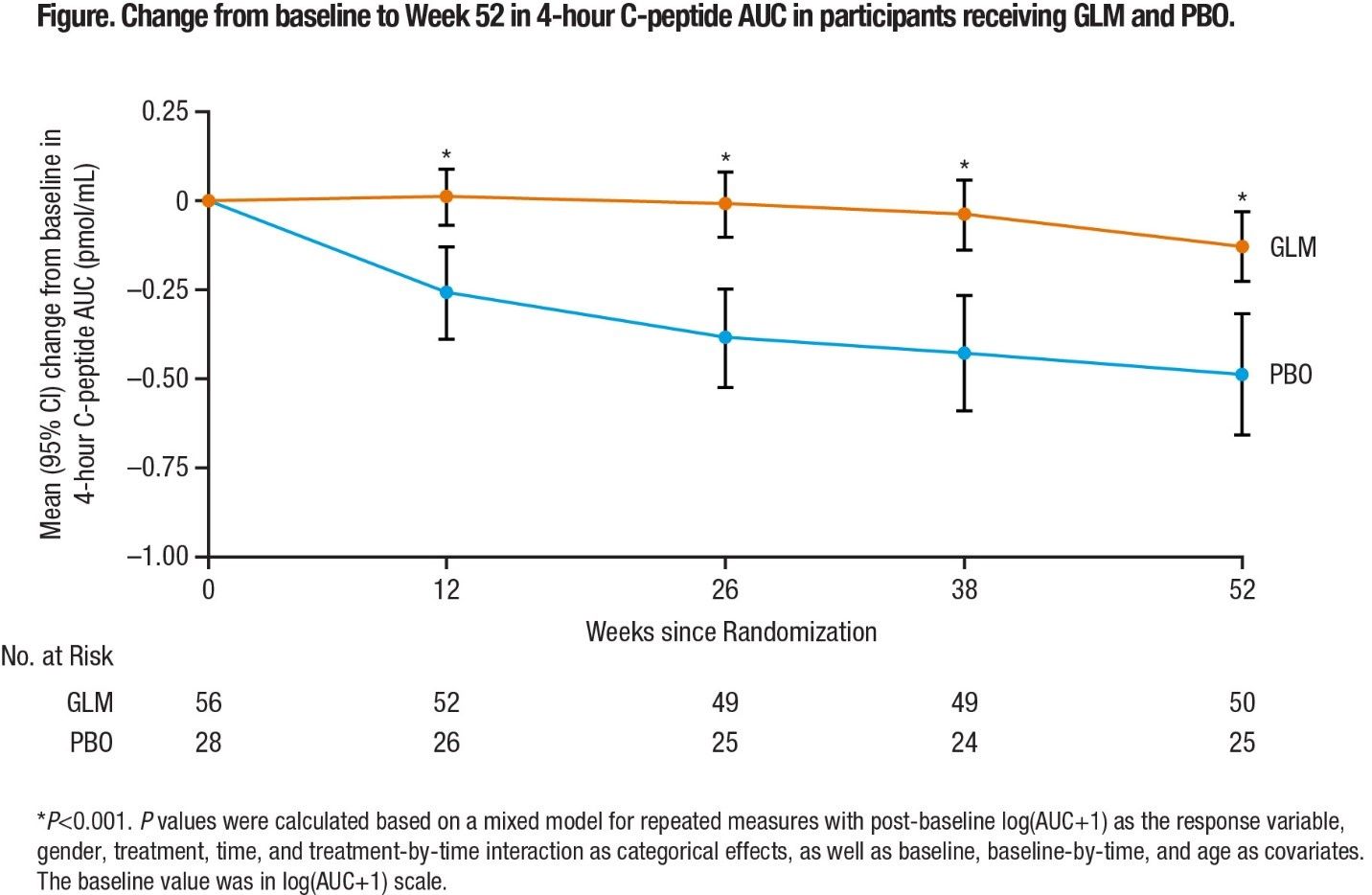
Source: American Diabetes Association
Benefits of Switching to Once-Weekly Semaglutide
Patients with T2D who switched to semaglutide, a GLP-1 receptor agonist from Novo Nordisk, from other GLP-1 receptor agonists saw greater A1C reductions and lost more weight after 6 months and were able to maintain those improvements after a year, according to an examination of claims data presented at the ADA Scientific Sessions.3
In the EXPERT study, researchers use the Explorys (IBM Watson) database to examine records from patients at least 18 years old who had at least 1 prescription for semaglutide after being prescribed a different GLP-1 receptor agonist therapy within the prior year. Both A1C and weight measurements were taken within 90 days before the index date.
Switching to semaglutide was associated with average A1C reductions of 2.2% for people with A1C above 9% before taking the drug, and reductions of 1.1% for those with A1C levels above 7%. The average weight loss was 2.2 kg at 6 months, rising to an average of 3.5 kg at 12 months, for both groups (Figure 2).
"More than half of people with type 2 diabetes do not reach their blood sugar target, yet we know that consistently poor blood sugar control can lead to serious complications," said Mads Krogsgaard Thomsen, executive vice president and chief science officer of Novo Nordisk in a statement. "Real-world data is therefore essential to help physicians select optimal treatment for their patients to meet their blood sugar goals.”
References
1. Rosenstock J, Ampudia-Blasco F, Lubwama R, et al. Real-world evidence of the effect on glycemic control with relatively simultaneous vs. sequential initiation of basal insulin and GLP-1 receptor agonists. Presented at: the 80th American Diabetes Association Scientific Sessions; June 12-16, 2020. Abstract 965-P.
2. Quattrin T, Haller MJ, Steck A, et al. Golimumab (GLM) preserves ß-cell function and reduces insulin use and hypoglycemia in children and young adults with recently diagnosed type 1 diabetes (T1D): the phase 2 TIGER study. Presented at: the 80th American Diabetes Association Scientific Sessions; June 12-16, 2020. Abstract 3-LB.
3. Lingvay I, Kirk AR, Lophaven S, et al. GLP-1 experiences patients switching to once-weekly semaglutide in a real-world setting (EXPERT Study). Presented at: the 80th American Diabetes Association Scientific Sessions; June 12-16, 2020. Abstract 954-P.
Exploring Pharmaceutical Innovations, Trust, and Access With CVS Health's CMO
July 11th 2024On this episode of Managed Care Cast, we're talking with the chief medical officer of CVS Health about recent pharmaceutical innovations, patient-provider relationships, and strategies to reduce drug costs.
Listen
