- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Spinal Muscular Atrophy: An Update for Managed Care Pharmacists
Abstract
The cost burden of patients with SMA is considerable, and is estimated to be approximately $4 million to $5 million over 10 years in patients with early-onset SMA. This cost is 54.2 times greater than an otherwise healthy population. The utilization of medication, resources, and cost differs between different types of SMA and is more intensive in infantile-onset SMA type 1. Patients often require supportive physical aides, ventilation, and other services to treat sequalae of muscle weakness. Early diagnosis and treatment initiation are necessary to maximize benefit of treatment. Genetic newborn screening has allowed for early diagnosis. With the approval of novel pharmacotherapy options for SMA, timely treatment initiation may help to decrease healthcare burden and costs associated with early-onset SMA. Current options are effective in improving mobility, but maximum benefit has yet to be seen as this population is still growing. Due to the cost of treatment, managed care pharmacists should consider appropriate utilization management and innovative outcomes-based payment models to decrease risk while maximizing outcomes.
Am J Manag Care. 2020;27(suppl 1):S13-S18. https://doi.org/10.37765/ajmc.2021.88593
Importance of Early and Effective Treatment in Spinal Muscular Atrophy
Cost of Care
The cost burden of patients with spinal muscular atrophy (SMA) is significant and varies due to the type of SMA and medications used. In a study conducted from January 2006 to March 2016 (before approval of pharmacologic therapy), investigators evaluated healthcare resource utilization and costs in patients. A total of 341 patients with SMA included in the analysis were stratified by infantile-onset SMA before age of 6 months (mean age, 0.2 years; n = 22), childhood-onset SMA between 6 months and 3 years of age (mean age, 0.9 years; n = 22), and late-onset SMA, which included all other patients with SMA (mean age, 52.2 years; n = 296), then were compared with a cohort without SMA. Medications that these patients used during their follow-up period were consistent with physical symptoms of SMA, such as gastroesophageal reflux disease, respiratory tract infections, muscle spasm, difficulty moving muscles and joints, and concurrent mental health disorders. Medications that have been used most commonly were acid suppressants, antibiotics, muscle relaxants, benzodiazepines, prokinetic agents, and anxiolytics. Of note, those with infantile SMA have observed higher rates of acid suppressants and prokinetic agents, whereas those of older-onset SMA had more use of muscle relaxants, benzodiazepines, anxiolytics, and antidepressants. Although these medications are generically available and inexpensive, there is an increased burden of higher medication usage. These medications are summarized in Table 1.1
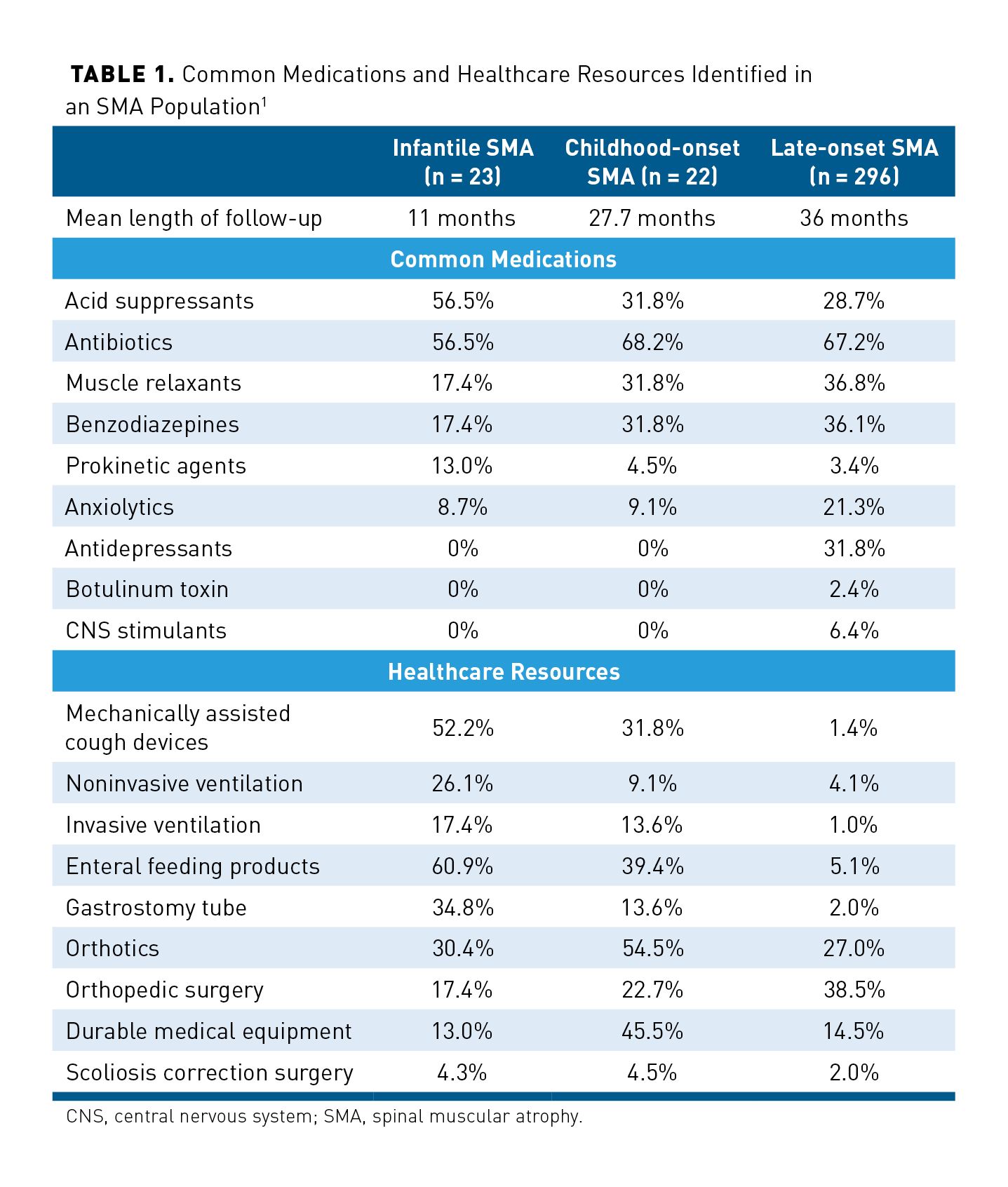
Along with increased medication usage, there was a significant number of patients with SMA who required utilization of costly resources. More patients with infantile SMA used a mechanically assisted cough device compared with late-onset SMA (52.2% vs 1.2%). Other healthcare resources that were highly utilized in infantile SMA included noninvasive ventilation (26.1%), invasive ventilation (17.4%), and gastrostomy tube (34.8%). A summary of common healthcare resources utilized from this study is shown in Table 1.1
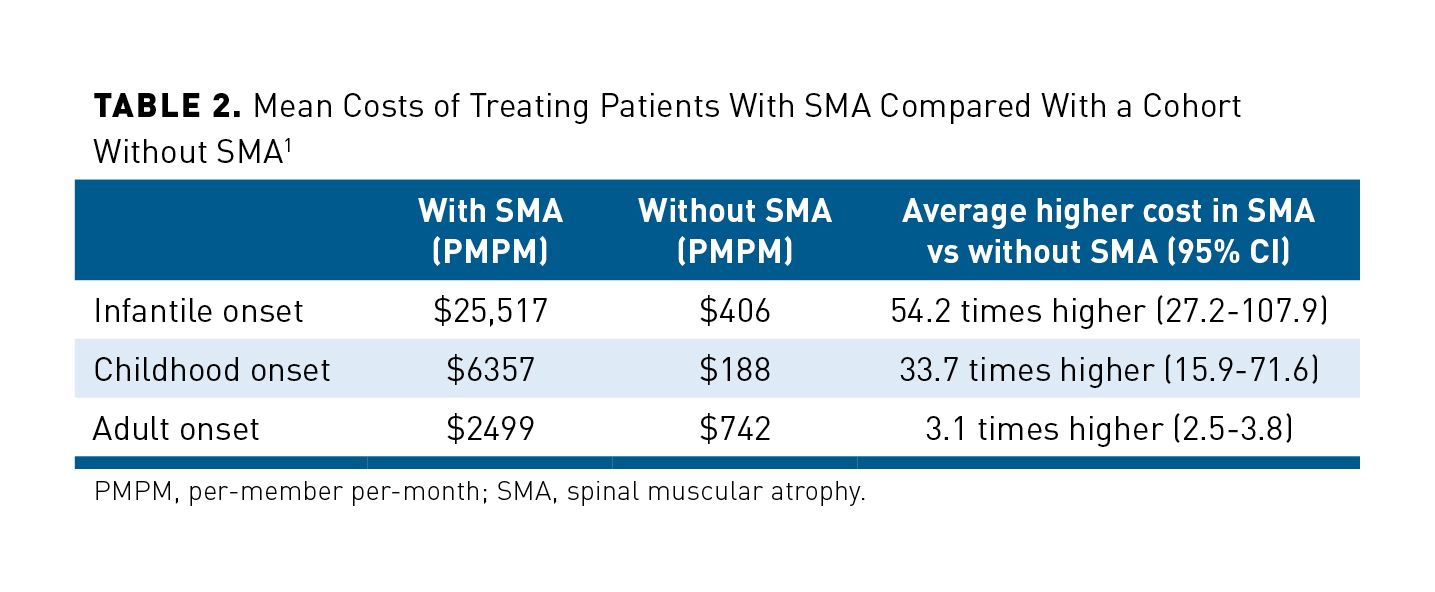
The high rates of medication usage and healthcare resource utilization translate into high costs associated with SMA. On a per-member per-month (PMPM) basis, those in the infantile SMA group had the highest mean costs ($25,517) followed by childhood onset ($6357) and late onset ($2499). In the matched cohort without SMA, the cost was significantly lower in the infantile group ($406), childhood onset ($188), and late onset ($742). A comparison of these costs is outlined in Table 2.1 It is also important to note that the average cost to treat a patient with infantile-onset SMA was 54.2 times higher than someone without SMA during a similar follow-up period; childhood onset was 33.7 times higher; and adult onset was 3.1 times higher.1
In a separate Institute for Clinical and Economic Review (ICER) evaluation of nusinersen and onasemnogene abeparvovec-xioi for SMA, evaluators estimated the annual cost of best supportive care (BSC) for SMA to be approximately $167,400 excluding any FDA-approved pharmacologic therapy. This is similar to other estimates of cost of care associated with routine SMA care.2 In a 2010 evaluation of total annual costs, those with early-onset SMA (<3 years old) had an estimated cost of $184,647 per patient compared with other SMA at $45,750 a year. Of the costs associated with early-onset SMA, $115,223 were directly attributable to medical costs. Total nonmedical costs were estimated to be $51,655 for this early-onset SMA population. When broken down by individual costs, professional caregiving made up a majority of annual costs ($50,542), followed by moving/home modification ($3685), other nonmedical costs such as food and travel ($2106), and purchasing/modifying transportation ($1814).3 It should be noted that these cost estimates were prior to FDA-approved pharmacotherapy options for SMA. Cost estimates were higher for SMA type 1 due to extensive healthcare needs and disease severity compared with later-onset SMA. This high healthcare burden on patients with early-onset SMA underlies the essential need for effective and early management of symptoms, which may have the potential to decrease costs associated with these symptoms.
Disease Progression and Need for Timely Diagnosis and Treatment
Left untreated, SMA results in significant comorbidities and mortality. Depending on the type of SMA (type 0-4), age of onset and clinical presentation will vary. In those with untreated SMA type 1, patients may never sit/stand and death will likely occur within the first 2 years of life. These patients suffer from physical symptoms of reduced muscle tone, decreased limited movements, difficulty with eating and swallowing, and impaired breathing that can lead to respiratory depression. In patients with SMA type 2, life expectancy is longer (young adult) than type 1 but also results in the need for mobility aids as well as potential need for ventilation. Those with type 3 have the potential to live a typical lifespan with treatment but have an increased risk of respiratory infections and musculoskeletal concerns, such as scoliosis and muscle/joint weakness. Those with SMA type 4 usually have mild-moderate physical symptoms and a normal lifespan.4 Historically, there has been a delay in SMA diagnosis of 3.6, 14.3, and 43.6 months for SMA types 1, 2, and 3, respectively.5 This delay in diagnosis may lead to irreversible disease progression and worsening prognosis. It is thought the delay occurs due to symptoms varying widely in onset and severity and can resemble other diseases. Additionally, the lack of expertise in the field can lead to healthcare professionals ruling out other diagnoses before considering SMA.
To mitigate these detrimental effects and high mortality rate, early diagnosis and appropriate treatment are essential. Genetic testing for SMA due to homozygous deletion of exon 7 in SMN1 has been added to the Recommended Uniform Screening Panel (RUSP). The RUSP is a list of disorders that the Secretary of the Department of Health and Human Services recommends adding to each state’s universal newborn screening (NBS) program. Disorders are added to the list if there is (1) a net benefit for screening, (2) the ability to screen for the disorder, and (3) the availability of effective treatments. Non-grandfathered health plans are also required to cover screenings for conditions listed on the RUSP without a co-payment, co-insurance, or deductible.6 As of 2020, 28 states had incorporated SMA into routine NBS, and 5 states have implemented SMA NBS pilot programs as a result of the RUSP guidelines.7
If newborns are positively screened for SMA types 0-3, patients should receive immediate treatment if pre-symptomatic. In those with SMA type 0, physician discretion should be used if the patient presents with symptoms. The effective treatment of patients before symptom manifestation may help to maintain motor neurons and preserve muscle motor function.8,9 This efficacy was demonstrated in clinical trials, but the true lifelong benefit of treatment has yet to be seen because the first pharmacotherapy option was approved in 2016. Recent releases of long-term safety and efficacy checkpoints do show continued durability of treatments. In clinical trials, all currently approved FDA therapies for SMA observed more benefit in patients treated earlier in their disease pathway.10 Current pharmacotherapy is approved in pediatrics and adult patients, but clinical trials have not included those with SMA type 4 (older onset). Before the approval of these therapies, disease management consisted of symptomatic management (eg, muscle relaxants, antibiotics), mobility aides (eg, walker, wheelchair), and other therapies. It is important to have open communication and coordination among the managed care organization and provider support staff to ensure timely treatment. Newborns have up to 30 days to be added to insurance benefits of parents and coverage will be backdated to the newborn day of birth. Additionally, under the Health Insurance Portability and Accountability Act, if enrolled within 30 days of birth, insurance companies may not impose preexisting condition exclusions on the newborn.11
Costs Associated With Treatment of SMA
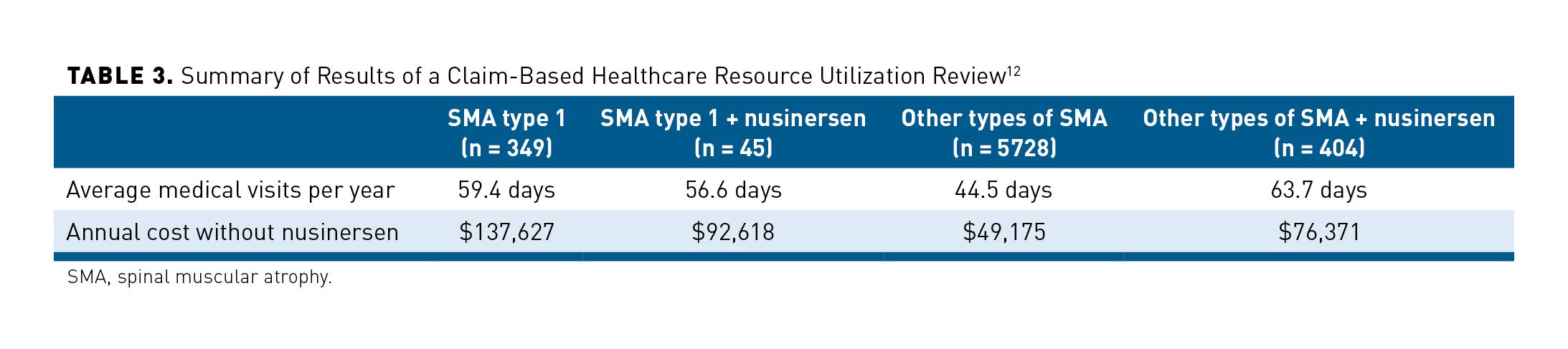
In 1 claim-based healthcare resource utilization review of patients with SMA from September 1, 2016 to August 31, 2018, patients were categorized into 4 cohorts: (1) patients with SMA type 1, (2) patients with SMA type 1 on nusinersen, (3) patients with other types of SMA, and (4) patients with other types of SMA on nusinersen. When the cost of nusinersen was excluded, the annual cost of treating patients with SMA type 1 (no nusinersen) was $137,627 compared with $92,618 in the SMA type 1 on nusinersen group. This may have been associated with a decrease in annual inpatient days of 14.1 and 4.6, respectively. However, this decrease in medical visits per year and annual cost without nusinersen was not consistent with other types of SMA. Authors noted limitations to their results, which included potentially underestimated costs that were not in the claims system. While limited, these data support that for those with SMA type 1, treatment of nusinersen reduced healthcare resource utilization and other total costs. These cost savings were offset by the real-world cost of nusinersen, which was $191,909 PMPM for the first 3 months (loading dose) followed by $36,882 PMPM for maintenance costs. The study results are summarized in Table 3.12
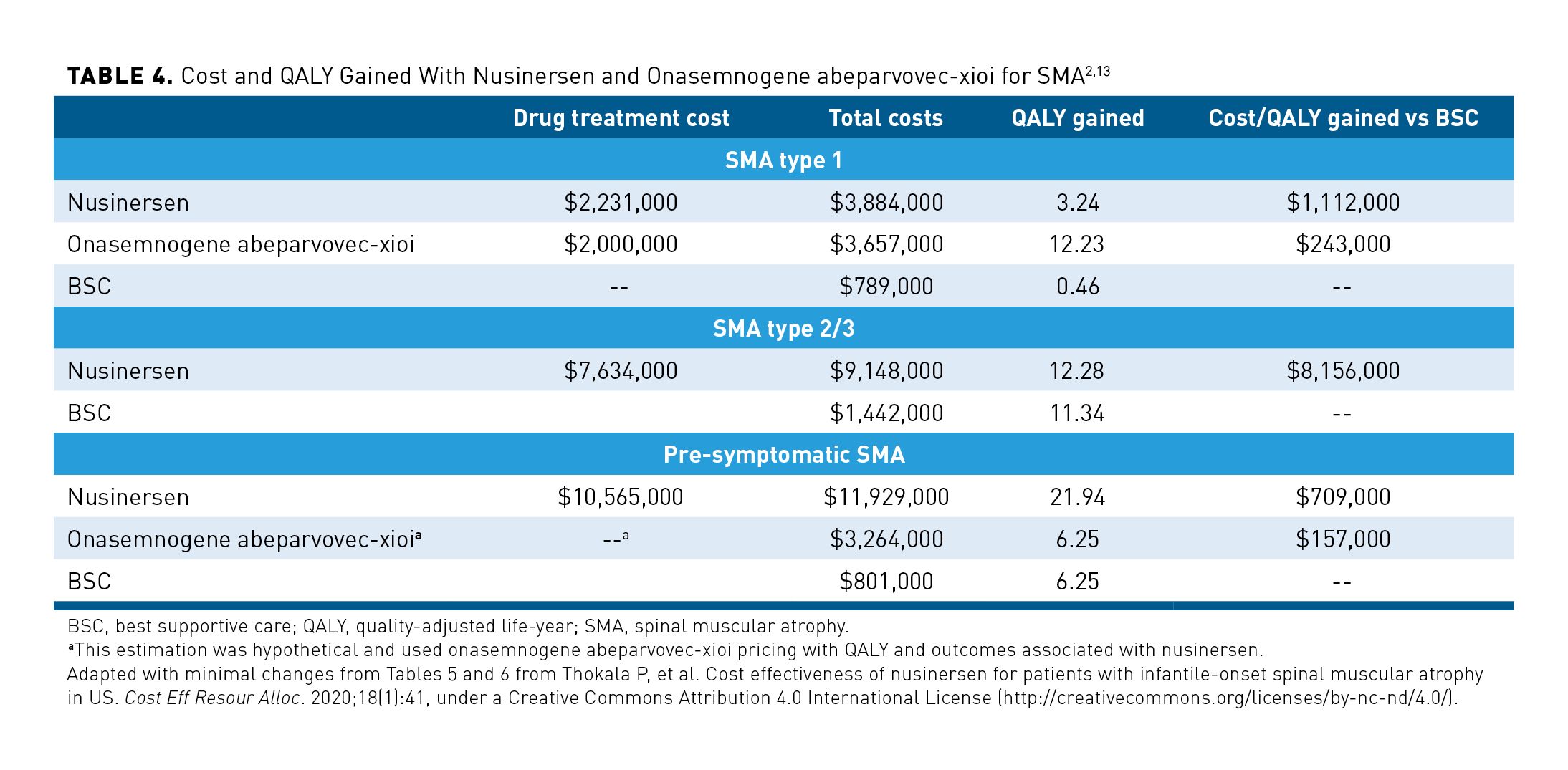
In an ICER review of nusinersen and onasemnogene abeparvovec-xioi for SMA, costs per quality-adjusted life-year (QALY) gained were described by type of SMA and intervention. For SMA type 1, the total cost of treatment with nusinersen and nontreatment healthcare costs were $3,884,000 compared with $789,000 for BSC. Nusinersen use was associated with total QALY gained of 3.24 years, total life-year (LY) gained of 7.64 years, and cost/QALY of $1,112,000. This is in comparison with onasemnogene abeparvovec-xioi, which had a relatively similar total cost of care of $3,657,000, a total of 12.23 QALY gained, 18.17 LY gained, and a cost/QALY gained of $243,000. It should be noted that this was before the approval of risdiplam, the other medication approved for the treatment of SMA. These results are summarized in Table 4.2,13
Researchers from this ICER analysis concluded that neither nusinersen nor onasemnogene abeparvovec-xioi met $150,000 per QALY gained to meet traditional cost-effectiveness thresholds. Nusinersen was the most cost-effective in the pre-symptomatic population, but the cost would still need to be reduced to $65,000 a year for it to meet traditional cost-effectiveness.2 When interpreting the cost-effectiveness of ultra-rare disorders, it is important to consider that traditional thresholds of cost per QALY gained may need to be reconsidered. With these novel treatments for ultra-rare disorders, higher costs for QALY gained may become more common.
In a separate study, authors examined the cost-effectiveness of onasemnogene abeparvovec-xioi and nusinersen. This study found an LY gain of 37.20 for onasemnogene abeparvovec-xioi and 9.68 LY for nusinersen. This was considerably higher than the ICER estimations of 18.17 LY for onasemnogene abeparvovec-xioi and 7.64 LY for nusinersen.14 It should be noted that, due to the one-time administration, the longer a patient’s life, onasemnogene abeparvovec-xioi becomes more cost-effective. Because we have yet to see the full benefit and lifespan of these patients, the full cost-effectiveness of treatment is to be determined. Managed care pharmacists should be aware of ongoing long-term safety and efficacy follow-up studies in patients who were administered onasemnogene abeparvovec-xioi to continuously update their understanding on the cost-benefit of gene therapy compared with more traditional medications. As favorable long-term safety and efficacy data continue to become available, it is expected to have an adjusted impact on the cost-effectiveness of SMA treatments.
Limited data are available regarding the cost-effectiveness of risdiplam for the treatment of SMA. However, treatment strategies should follow the same strategy as other available agents. Early diagnosis and initiation of therapy as soon as possible prevent loss of neurons and muscle function. Potential advantages with risdiplam include an oral formulation that would not need to be administered by a healthcare professional.
Managed Care Considerations for the Management of SMA
At this time, ICER could not identify any opportunities to reduce unnecessary costs for patients with SMA. However, because of high drug costs and uncertain long-term efficacy and safety, managed care organizations (MCOs) should develop appropriate coverage criteria based on the most current clinical evidence as well as guidelines and input from local providers. Of note, a set of recommendations regarding coverage criteria for SMA pharmacotherapy was proposed. The considerations include2:
Diagnosis: Should be confirmed via genetic testing, without the need for more than one genetic test.
Pre-symptomatic SMA: Through genetic testing, patients can be screened for number of SMN2 copies where 4 or more copies may predict less severe forms of SMA. Current recommendations support the immediate initiation of pharmacotherapy in patients who test for 4 or fewer copies of SMN2.
Age: Pre-symptomatic patients should begin therapy immediately. Evidence does not support loss of efficacy in patients with SMA type 2-3 at all ages. Therefore, there should not be an age restriction for use.
Other clinical criteria: Pre-symptomatic patients should not have any additional requirements. It is debatable if patients who are immobilized or with more severe symptoms should be eligible for treatment. Some MCOs may also place restrictions on patients who are already on permanent ventilation. However, advocates have noted that small motor improvements can result in large quality-of-life gains in patients.
Renewal criteria: A baseline assessment should be documented using a standard outcome measure. This baseline can serve as a guideline for evaluation of disease stability or improvement. For nusinersen and risdiplam, MCOs may require documentation of treatment success needed for continued approvals. Every patient is unique, and the provider managing the patient should assess if there has been improvement or at least a halt of disease progression to justify continued approvals.
Combination or sequential therapy: MCOs should monitor for concurrent use of FDA-approved treatments for SMA and closely monitor switching between agents if an inadequate response is seen in one therapy. Implications for switching should be evaluated and considered on a case-by-case basis in the current absence of strong clinical data on the subject.
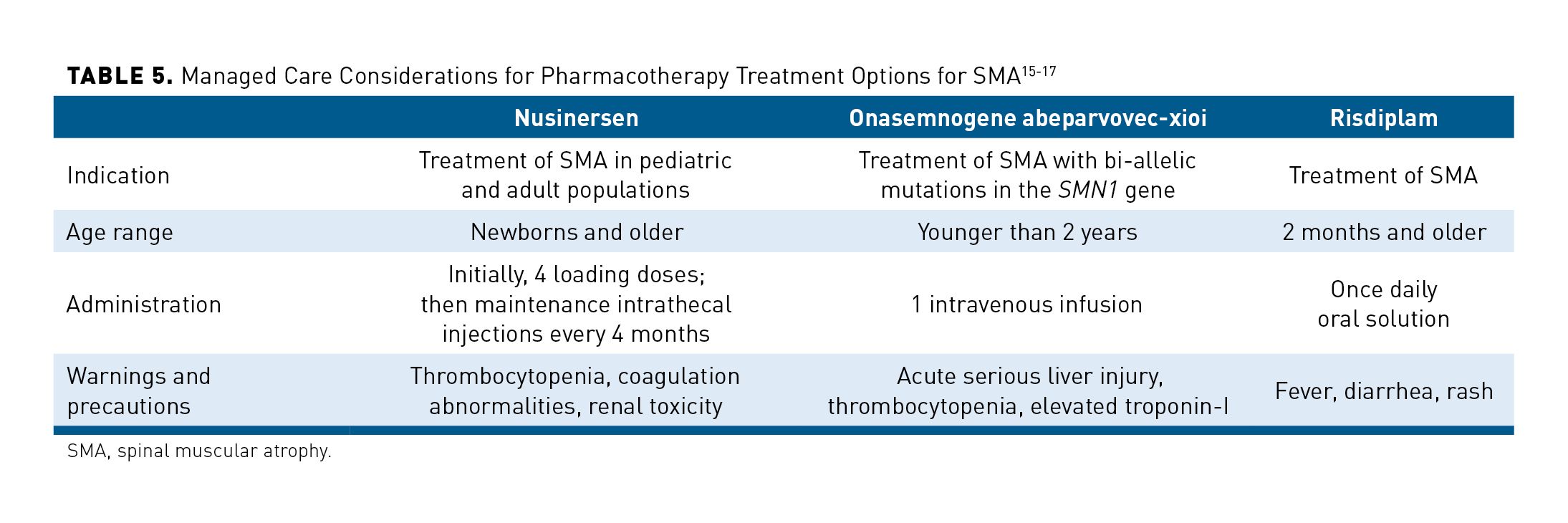
When developing coverage criteria for medications used for SMA, managed care pharmacists should also take into consideration drug-specific characteristics, such as indication, approved age range, administration concerns, and significant safety concerns. Table 5 summarizes these considerations.15-17 Considerations for nusinersen include evaluation for spinal deformities, planning for regular intrathecal administration, and coordination with the patient to ensure access to a location for appropriate administration. Costs of these administrations should consider things like transportation and lodging, especially in geographically isolated patients. While risdiplam has fewer administration concerns (oral solution), it lacks long-term safety and efficacy data and is only indicated for patients 2 months and older. Onasemnogene abeparvovec-xioi is administered once and is only indicated for patients who are younger than 2 years and meet eligibility requirements. Patients with late-onset SMA would not fit into this demographic.
Because of the high cost of treatment with onasemnogene abeparvovec-xioi, and unknown long-term efficacy, MCOs may consider an outcomes-based payment contract with the manufacturer. By establishing an outcomes-based payment contract, the MCO can transfer some of the financial risk back to the manufacturer if outcomes are insufficient.18 However, these outcomes should be quantifiable, widely accepted outcomes. With SMA, a challenge for implementing an outcomes-based payment contract will be determining what is an appropriate outcome response, and what constitutes an inadequate response. By tying financial risk or health outcomes, MCOs can hold manufacturers to their efficacy data in a real-world setting.2,19
In an example of this outcomes-based payment contracting, the manufacturer of onasemnogene abeparvovec-xioi offers an option to pay over multiple years and engage in outcomes-based payments to bring the overall medical cost to within a more cost-effective range.20 As this payment model is still novel, MCOs should pay extra attention to how payments are made and how outcomes have affected payments overall. Future studies on how this outcomes-based payment model has affected overall cost-effectiveness will help to make future formulary determinations.
Regardless of treatment selection, coverage criteria should consider FDA-approved indication, current efficacy, safety data, and diagnosis based on genetic testing. As evidence continues to develop, managed care pharmacists should strive to incorporate the most updated recommendations into coverage criteria. For example, in a recent update to SMA diagnosis, immediate pharmacotherapy is recommended for infants who screened positive for 4 copies of SMN2 (indicative of SMA type 3 or 4).8 Managed care pharmacists should focus on minimizing inappropriate utilization and maximizing treatment potential through encouragement of early diagnosis and treatment.
Once an appropriate treatment has been determined, it is important to have effective coordination between the medical and pharmacy benefit. Current SMA treatments offer a unique challenge in that the 3 current products have the potential for 3 unique methods of coverage and payment. Risdiplam as an oral therapy would be processed and evaluated for medical necessity under the pharmacy benefit that is managed by a pharmacy benefits manager. Nusinersen requires administration by a healthcare professional and would typically be evaluated and covered under the medical benefit managed by a medical payer or third-party administrator. Onasemnogene abeparvovec-xioi as a one-time infusion would be processed under the medical benefit with the required inpatient costs associated with administration. Given the various stakeholders involved, lack of coordination has the potential to result in mixed messaging of approval or denial due to the differences in criteria by the different vendors.
Conclusions
The cost burden of early-onset SMA is significant and can cost approximately $184,647 per patient per year compared with other types of SMA at $45,750 a year. The significantly higher costs associated with earlier-onset SMA can be attributed to increased need for extensive physical supportive care and medical needs. Advances in the ability to genetically screen for SMA, as well as the availability of novel treatment options that should be started before symptom development, offer MCOs and providers an opportunity to address patients with SMA like never before. By treating SMA before symptom development, there is a potential to mitigate disease progression and improve patient outcomes. Current pharmacotherapy is recommended in patients who are pre-symptomatic at all types of SMA. Each treatment option has a different route of administration, method of payment, and potential advantages. Regarding cost-effectiveness, it was estimated that onasemnogene abeparvovec-xioi has a more favorable cost per QALY gained compared with nusinersen in pre-symptomatic type 1 SMA, but neither were determined to be cost-effective when compared with the $150,000 per QALY-gained threshold. This benefit is augmented with increased lifespan in patients. Managed care pharmacists should take into consideration long-term safety and efficacy data as they are published. Utilization management of these medications should be in accordance with current guidelines and best available evidence. An outcomes-based payment contract should be considered for onasemnogene abeparvovec-xioi to decrease financial risk and aim for optimal health outcomes.
Author affiliation: Dr Pannier is Head of Clinical Services at SmithRx in Salt Lake City, UT.
Funding source: This activity is supported by an educational grant from Biogen.
Author disclosure: Dr Pannier has no relevant financial relationships with commercial interests to disclose.
Author information: Concept and design; drafting of the manuscript; critical revision of the manuscript for important intellectual content; final approval of manuscript.
Address correspondence to: alanpannier@gmail.com
Medical writing and editorial support provided by: Andrew Abe, PharmD.
REFERENCES
1. Tan H, Gu T, Chen E, Punekar R, Shieh PB. Healthcare utilization, costs of care, and mortality among patients with spinal muscular atrophy. J Health Econ Outcomes Res. 2019;6(3):185-195. doi: 10.36469/63185
2. Institute for Clinical and Economic Review (ICER). Final evidence report. Spinraza and Zolgensma for spinal muscular atrophy: effectiveness and value. April 3, 2019. Updated May 24, 2019. Confidential data unmasked November 2, 2020. Accessed November 18, 2020. https://icer-review.org/wp-content/uploads/2018/07/ICER_SMA_Final_Evidence_Report_110220.pdf
3. Lewin Group. Cost of Amyotrophic Lateral Sclerosis, Muscular Dystrophy, and Spinal Muscular Atrophy in the United States. March 12, 2012. Accessed January 21, 2021. www.mda.org/sites/default/files/Cost_Illness_Report.pdf
4. Spinal Muscular Atrophy Fact Sheet | National Institute of Neurological Disorders and Stroke. Page modified October 8, 2020. Accessed November 18, 2020. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Spinal-Muscular-Atrophy-Fact-Sheet
5. Lin CW, Kalb SJ, Yeh WS. Delay in diagnosis of spinal muscular atrophy: a systematic literature review. Pediatr Neurol. 2015;53(4):293-300. doi: 10.1016/j.pediatrneurol.2015.06.002
6. Recommended Uniform Screening Panel. US Health Resources & Services Administration. Published July 3, 2017. Page reviewed February 2020. Accessed November 18, 2020. www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html
7. McCall S. Newborn screening for spinal muscular atrophy. Cure SMA. Accessed December 16, 2020. www.curesma.org/newborn-screening-for-sma/
8. Glascock J, Sampson J, Connolly AM, et al. Revised recommendations for the treatment of infants diagnosed with spinal muscular atrophy via newborn screening who have 4 copies of SMN2. J Neuromuscul Dis. 2020;7(2):97-100. doi: 10.3233/JND-190468
9. Glascock J, Sampson J, Haidet-Phillips A, et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145-158. doi: 10.3233/JND-180304
10. Dangouloff T, Servais L. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: current perspectives. Ther Clin Risk Manag. 2019;15:1153-1161. doi: 10.2147/TCRM.S172291
11. Protections for Newborns, Adopted Children, and New Parents...The Newborns’ and Mothers’ Health Protection Act of 1996 | U.S. Department of Labor. Accessed December 16, 2020. www.dol.gov/agencies/ebsa/about-ebsa/our-activities/resource-center/publications/protections-for-newborns
12. Droege M, Sproule D, Arjunji R, Gauthier-Loiselle M, Cloutier M, Dabbous O. Economic burden of spinal muscular atrophy in the United States: a contemporary assessment. J Med Econ. 2020;23(1):70-79. doi: 10.1080/13696998.2019.1646263
13. Thokala P, Stevenson M, Kumar VM, Ren S, Ellis AG, Chapman RH. Cost effectiveness of nusinersen for patients with infantile-onset spinal muscular atrophy in US. Cost Eff Resour Alloc. 2020;18(1):41. doi: 10.1186/s12962-020-00234-8
14. Malone DC, Dean R, Arjunji R, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7(1):1601484. doi: 10.1080/20016689.2019.1601484
15. Spinraza. Prescribing information. Biogen; 2020. Accessed January 20, 2021. www.spinraza-hcp.com/content/dam/commercial/spinraza/hcp/en_us/pdf/spinraza-prescribing-information.pdf
16. Zolgensma. Prescribing information. AveXis; 2019. Accessed January 20, 2021. www.avexis.com/us/Content/pdf/prescribing_information.pdf
17. Evrysdi. Prescribing information. Genentech; 2020. Accessed January 20, 2021. www.gene.com/download/pdf/evrysdi_prescribing.pdf
18. Value & Outcomes-Based Contracting. Pharmaceutical Care Management Association (PCMA). Accessed November 20, 2020. www.pcmanet.org/policy-issues/value-outcomes-based-contracting/
19. Vlaanderen FP, Tanke MA, Bloem BR, et al. Design and effects of outcome-based payment models in healthcare: a systematic review. Eur J Health Econ. 2019;20(2):217-232. doi: 10.1007/s10198-018-0989-8
20. Rosenberg J. FDA approves gene therapy with $2.1M price tag for spinal muscular atrophy in pediatric patients. AJMC. May 24, 2019. Accessed November 20, 2020. www.ajmc.com/view/fda-approves-gene-therapy-with-21m-price-tag-for-spinal-muscular-atrophy-in-pediatric-patients
