- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Semaglutide 2.4-mg Injection as a Novel Approach for Chronic Weight Management
ABSTRACT
Anti-obesity medications used with lifestyle intervention produce greater and more sustained weight loss than does lifestyle intervention alone. However, until 2021, FDA-approved medications for the long-term treatment of obesity in the general adult population had not demonstrated the sustained loss of 15% body weight needed to meet or exceed all guideline-recommended targets for weight-related complications. To meet this need, investigators launched the Semaglutide Treatment Effect in People with obesity (STEP) program of phase 3 clinical trials to assess the safety and efficacy of a weekly 2.4-mg subcutaneous injection of semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA). Following the results of STEPs 1 to 4, the FDA approved semaglutide therapy in patients with obesity. This article examines the design, efficacy, and safety of semaglutide therapy as revealed by the results of STEPs 1 to 4. These trials included adults with obesity who reported at least 1 unsuccessful attempt to reduce body weight by means of diet. STEP 2 studied effects on patients who also had type 2 diabetes (T2D); STEPs 1, 3, and 4 excluded patients with this condition. STEP 3 examined the effect of this pharmacotherapy plus intensive behavioral therapy and a 2-step, intensively restricted dietary plan. STEP 4 assessed the effects of continuing semaglutide 2.4 mg vs switching to placebo after an initial treatment period. In the trials comparing the effects of semaglutide 2.4‑mg treatment with those of placebo across 68 weeks and in patients with no T2D (ie, STEPs 1 and 3), patients treated with semaglutide achieved a mean −14.9% to −16.0% weight change. This far exceeds the 4.0% to 10.9% weight loss seen with other approved antiobesity medications. In STEPs 2 and 4, the estimated treatment differences between the semaglutide 2.4-mg and placebo arms were −6.2% and −14.8%, respectively. Safety and tolerability of this treatment in STEPs 1 to 4 was consistent with those of other GLP-1RA–based therapies. Ultimately, the results of the first 4 STEP trials demonstrated that semaglutide 2.4 mg is a safe, well-tolerated, and highly effective treatment to promote weight loss, avoid weight regain, and mitigate the effects of the prevalent, chronic disease of obesity. In November 2022, based upon the results of STEPs 1 to 3 and other trials, the American Gastroenterological Association recommended that semaglutide 2.4 mg “be prioritized over other approved [anti-obesity medications] for the long-term treatment of obesity for most patients.”
Am J Manag Care. 2022;28(suppl 15):S297-S306. doi:10.37765/ajmc.2022.89293
For author information and disclosures, see end of text.
Introduction
In 2017 and 2018, approximately 42.4% of adults in the United States had obesity (defined as a body mass index [BMI] ≥ 30 kg/m2), according to data from the 2017-2018 National Health and Nutrition Examination Survey.1 Although a reduced-calorie diet, exercise, and behavioral modifications form the cornerstone of guideline-recommended obesity treatment, these lifestyle interventions alone are associated with moderate weight loss and with weight regain.2,3 Pharmacotherapy for weight loss in conjunction with lifestyle interventions produces greater weight loss sustained over time than does lifestyle intervention alone.2,3 Individuals with a BMI greater than 30 kg/m2 (ie, obesity) and those with a BMI of at least 27 kg/m2 (ie, overweight) who have a weight-related comorbidity should be considered for add-on treatment with pharmacotherapy.4 Bariatric surgery should be considered when therapeutic goals cannot be attained using structured lifestyle change and pharmacotherapy alone, or in conjunction with lifestyle therapy and pharmacotherapy.5,6
The FDA-approved medications for the long-term treatment of obesity have provided patients in the general adult population with mean weight loss from 4.0% to 10.9% of body weight after 1 year of treatment.7 A mean weight loss target of at least 5% is the lowest body weight reduction recommended to mitigate certain weight-related complications; however, achievement of greater weight loss is necessary for clinically significant improvements in some comorbidities.2 Weight loss of 15% would meet or exceed the recommended targets for all weight-related complications considered in the most widely accepted guidelines for obesity treatment.2 Therefore, there is a need for pharmacotherapeutic strategies that can provide sustained, long-term weight loss of 15%.
The glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1RA) class has emerged with the potential to meet this need. Several GLP-1RA medications, such as liraglutide (Victoza®; Novo Nordisk), have been FDA-approved for the treatment of type 2 diabetes (T2D).8-10 Liraglutide is also FDA-approved as a daily 3.0-mg injection to be used with lifestyle intervention for the treatment of patients with a BMI greater than 30 kg/m2 and for those with a BMI of at least 27 kg/m2 who have a weight-related comorbidity (Saxenda®; Novo Nordisk).11 Treatment with 3.0-mg liraglutide was associated with clinically significant weight loss in clinical trials; however, in a large (n = 3731), randomized clinical trial (RCT) assessing the impact of liraglutide 3.0 mg upon weight loss in patients with obesity, patients in the liraglutide arm experienced a mean weight loss of only 8.0% (SD, ± 6.7%) at 56 weeks.7
Semaglutide has 94% sequence homology to human GLP-1 and acts as a GLP-1RA that selectively binds to and activates the GLP-1R.12 It was initially approved by the FDA for the treatment of T2D as a weekly subcutaneous injection at doses up to 2 mg (Ozempic®; Novo Nordisk) and in tablet form at doses up to 14 mg daily (Rybelsus®; Novo Nordisk).13,14 However, in a 52-week, randomized, phase 2 trial of patients with obesity (BMI ≥ 30 kg/m2) but without T2D, daily treatment with semaglutide 0.4 mg was associated with greater mean weight loss than that associated with daily liraglutide 3.0 mg and placebo (–13.8% vs –7.8% and –2.3%, respectively).15
Based on these promising results, the Semaglutide Treatment Effect in People with obesity (STEP) program of phase 3 clinical trials was initiated to investigate the safety and efficacy of once-weekly subcutaneous semaglutide 2.4-mg injection for chronic weight management.16 In STEPs 1 (NCT03548935) and 3 (NCT03611582), use of semaglutide demonstrated a mean weight loss of −14.9% to −16.0% across a broad population of patients with obesity after 68 weeks.17,18
In 2021, based on the results of these 2 trials and those of STEPs 2 (NCT03552757) and 4 (NCT03548987), weekly semaglutide 2.4‑mg subcutaneous injection was FDA-approved as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adult patients with an initial BMI of 30 kg/m2 or greater or for patients with a BMI of 27 kg/m2 or greater in the presence of at least 1 weight-related condition.12,16,19
The Semaglutide Treatment Effect in People with Obesity (STEP) Program
The STEP program involves 7 completed and 5 ongoing phase 3, double-blind, randomized trials evaluating the safety and efficacy of semaglutide 2.4-mg subcutaneous injection as an adjunct to lifestyle therapy compared with either placebo, semaglutide 1.0 mg, semaglutide 1.7 mg, or liraglutide 3.0 mg (Table 1).16-18,20-33 Treatment efficacy and safety have been or are being studied over several time intervals, ranging from 44 weeks (STEP 7 [NCT04251156]) to 104 weeks (STEP 5 [NCT03693430]). One trial (STEP 4) compared the efficacy and safety of continued treatment with semaglutide 2.4 mg vs switch to placebo after initial treatment with semaglutide.
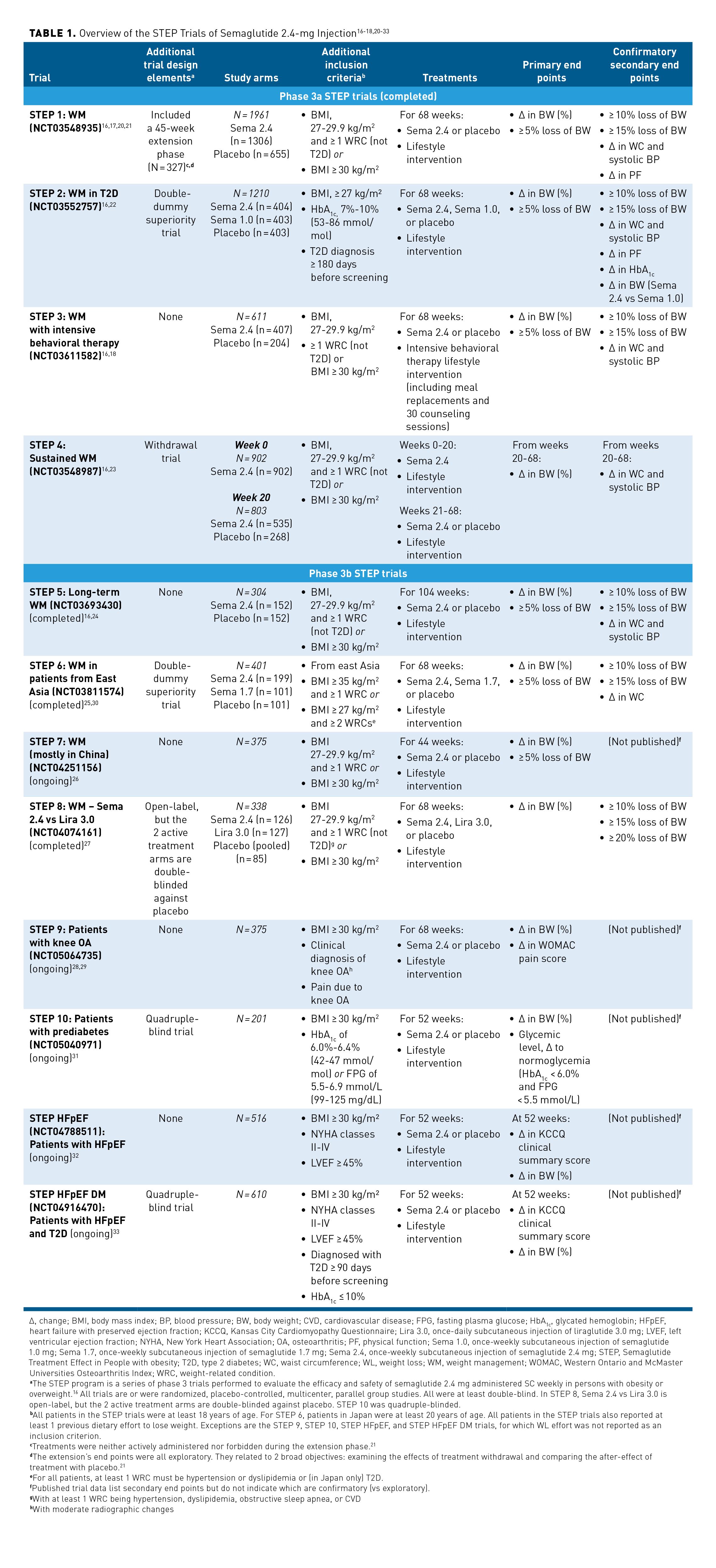
The STEP program enrolled patient populations with a range of comorbid conditions, including those with and without T2D; those with prediabetes, heart failure, or knee osteoarthritis; patients from East Asia; and individuals largely from China. STEP 1 also included an extension phase, which is not summarized within this article; the results were published after FDA approval (Table 1).16-18,20-33 This article reviews the study design and findings from the phase 3, randomized, placebo-controlled STEP 1, 2, 3, and 4 trials that were used for FDA approval of semaglutide.12,16,19
Trial Design for the STEP 1, 2, 3, and 4 Trials
For more information regarding the study design, treatment arms, inclusion/exclusion criteria, primary end points, and confirmatory secondary end points of the STEP 1, 2, 3, and 4 trials, please see Table 1.16-18,20-33
Patient Population
Across the 4 trials, patient enrollment criteria included an age of at least 18 years, at least 1 self-reported unsuccessful attempt to reduce body weight by means of diet, and a BMI of at least 27 kg/m2. Patients who had gained or lost over 5 kg (11 lb) in the 90 days leading up to screening were excluded from all trials. An additional exclusion criterion was treatment with an anti-obesity medication (AOM) during this 90-day period.16-18,22
Treatment Arms and Dosing
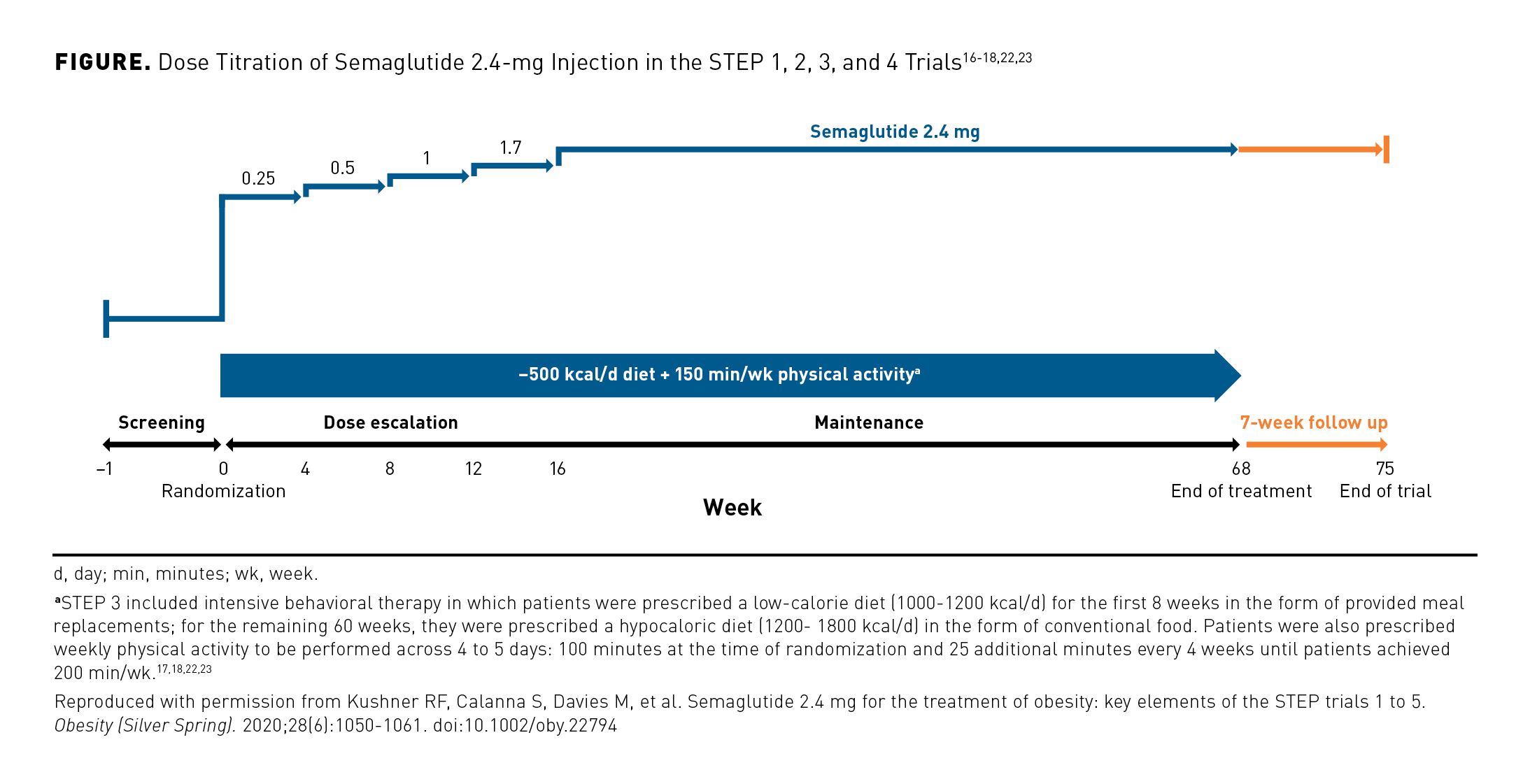
In STEPs 1 to 4, patients received lifestyle intervention in combination with either placebo, subcutaneous semaglutide 1.0 mg, or semaglutide 2.4 mg for 68 weeks. The trials included a 7-week follow-up period. In patients randomly assigned to receive semaglutide 2.4 mg, the medication was initiated at a dose of 0.25 mg. The dose was escalated every 4 weeks (0.5 mg, 1.0 mg, 1.7 mg, 2.4 mg) over a 16-week period; this was followed by a 52-week period at the maintenance dose of semaglutide 2.4 mg (Figure).16-18,22,23 STEPs 1 to 3 assessed outcomes across this 68-week treatment period. To determine the effects of semaglutide 2.4 mg on sustained weight management, patients in STEP 4 received semaglutide on a dose escalation schedule for 20 weeks and then were randomly assigned to either continue active treatment or switch to placebo for the duration of the study.16
Lifestyle Intervention
Lifestyle intervention included nonmonetary incentives to promote physical activity (eg, jump ropes and kettle bells), support from a multidisciplinary team that included a dietitian or similarly qualified provider, and periodic counseling.16 Lifestyle intervention for STEPs 1, 2, and 4 was defined as prescription of both a daily deficit of 500 kcal (relative to estimated caloric expenditure at randomization) and 150 min/wk of physical activity, plus counseling and support to bolster adherence to this regimen.16
Individual Trial Characteristics
Patients with T2D were excluded from STEPs 1, 3, and 4, which assessed semaglutide 2.4 mg compared with placebo as an adjunct to lifestyle intervention in a general population of patients with a BMI of at least 27 kg/m2 plus 1 or more weight-related (non-T2D) complications or a BMI of at least 30 kg/m2.16,17,23 STEP 2, which included patients with T2D and BMI of at least 27 kg/m2, assessed the superiority of semaglutide 2.4 mg over semaglutide 1.0 mg as well as over placebo. For patients receiving semaglutide 1.0 mg, the dose escalation period (0.5-1.0 mg every 4 weeks) ceased 8 weeks after treatment initiation.
Lifestyle intervention in STEP 3 was unique relative to that of the other trials in the STEP program. STEP 3 included an intensive behavioral therapy component consisting of a low-calorie diet (1000-1200 kcal/d) for the first 8 weeks in the form of provided meal replacements; for the remaining 60 weeks, they were prescribed a hypocaloric diet (1200-1800 kcal/d) in the form of conventional food. Patients were also prescribed weekly physical activity to be performed across 4 to 5 days: 100 minutes at the time of randomization and 25 additional minutes every 4 weeks until patients achieved 200 min/wk. Patients in STEP 3 also received 30 behavioral therapy sessions, which is approximately 13 more than received by participants in the other trials.17,18,22,23
Efficacy of Semaglutide 2.4 mg in STEPs 1 to 4
Weight Change Outcomes
The percentage change in body weight from baseline to end of treatment (EOT) was a primary end point across the trials. In STEP 4, baseline was week 20 (when patients were randomly assigned to continue semaglutide or switch to placebo) to assess the sustained effects of semaglutide 2.4 mg after an acute weight loss. Across STEPs 1 to 4, the mean percentage change in body weight from baseline to EOT was significantly greater in patients treated with semaglutide 2.4-mg injection vs those given placebo (P < .001 for all) (Table 2).16-18,22 The estimated treatment differences between the semaglutide 2.4 mg and placebo arms ranged from −6.2% (95% CI, −7.3% to −5.2%) in STEP 2 to −14.8% (95% CI, −16.0% to −13.5%) in STEP 4.22,23
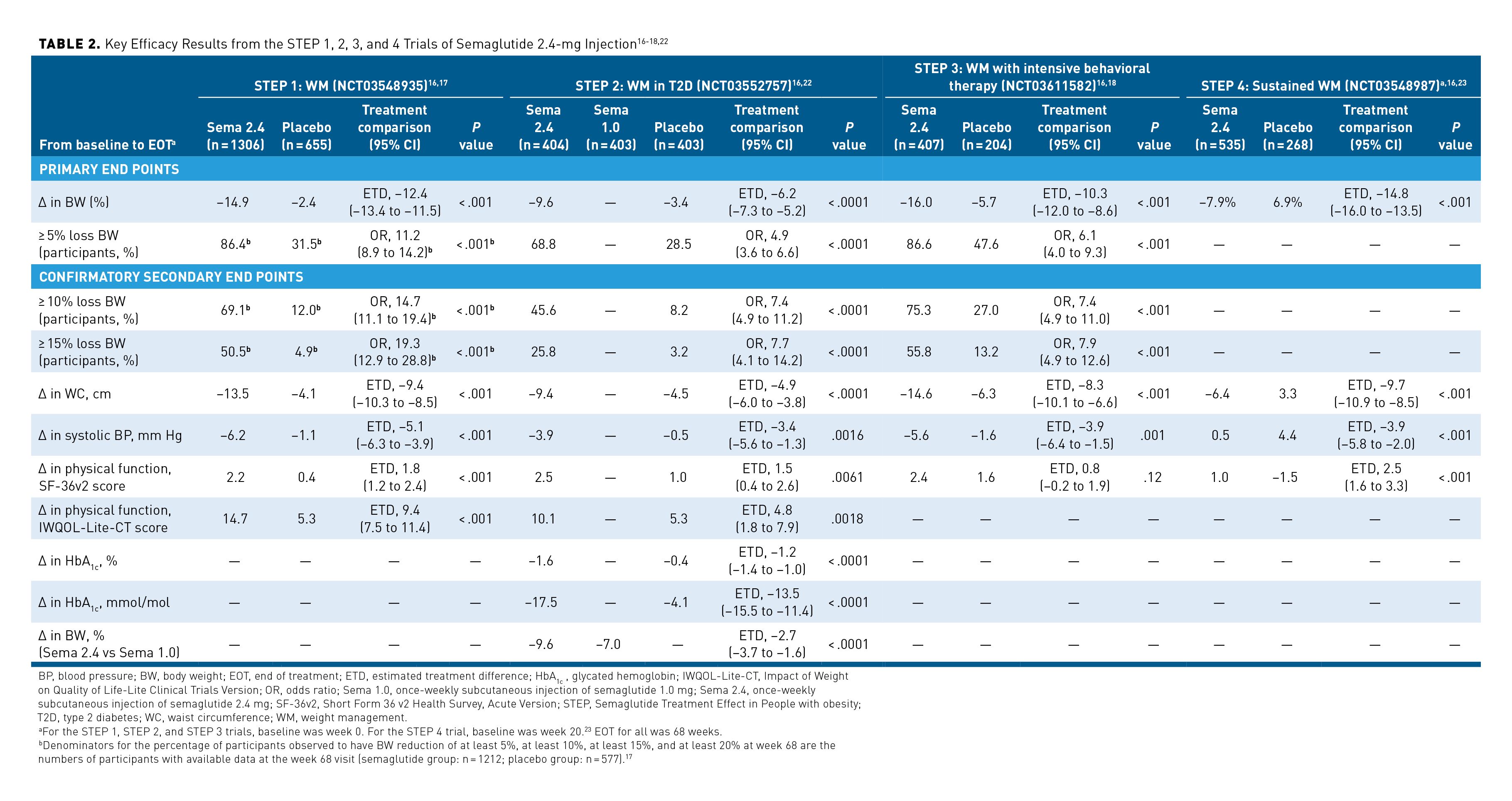
STEPs 1 to 3 included a co-primary end point of at least 5% weight loss from baseline to EOT. Semaglutide 2.4-mg treatment given for 68 weeks was associated with a significantly greater likelihood of 5% or greater body weight reduction vs placebo (P < .001 for all). Odds ratios (ORs) for achieving at least a 5% weight reduction with semaglutide vs placebo ranged from 4.9 (95% CI, 3.6-6.6) in STEP 2 to 11.2 (95% CI, 8.9-14.2) in STEP 1.17,18,22,23 STEPs 1 to 3 also included confirmatory secondary end points of at least 10% weight loss and of at least 15% weight loss from baseline to EOT. Patients treated with semaglutide 2.4 mg were significantly more likely to lose at least 10% and 15% of their body weight at EOT than were patients treated with placebo (P < .001 for all). ORs for 10% body weight loss ranged from 7.4 (95% CI, 4.9-11.0) in STEP 3 to 14.7 (95% CI, 11.1-19.4) in STEP 1. ORs for 15% body weight loss ranged from 7.7 (95% CI, 4.1-14.2) in STEP 2 to 19.3 (95% CI, 12.9-28.8) in STEP 1.
As a confirmatory secondary end point in STEP 2, change in body weight at EOT from baseline in the group receiving semaglutide 2.4 mg was compared with that in the group receiving semaglutide 1.0 mg.16-18,22 Significantly greater weight loss was achieved by patients given semaglutide 2.4 mg vs those given semaglutide 1.0 mg; the estimated treatment difference was −2.65 (95% CI, −3.66 to −1.64; P < .0001).22
These weight management results suggest that treatment with semaglutide 2.4 mg can help patients with obesity (both with and without T2D) achieve weight loss that meets or exceeds recommended targets.
Cardiometabolic Risk Outcomes
Changes in 2 cardiometabolic risk factors—waist circumference (cm) and systolic blood pressure (mm Hg)—were evaluated as confirmatory secondary end points across STEPs 1 to 4.16,17 Other cardiometabolic risk factors including diastolic blood pressure (mm Hg) and lipid levels were measured as supportive or exploratory end point; these results are not summarized here, as they were not controlled for multiplicity.17,18,22,23
Compared with patients given placebo, patients given semaglutide 2.4-mg treatment had significantly greater reductions in waist circumference from baseline to EOT across STEP trials 1, 2, 3, and 4 (P < .001 for all). The between-group differences in waist circumference ranged from −4.9 cm (95% CI, −6.0 to −3.8 cm) in STEP 2 to −9.7 cm (95% CI, −10.9 to −8.5) in STEP 4. Additionally, the semaglutide 2.4-mg arm experienced significant reductions in systolic blood pressure vs the placebo arm across these trials (P ≤ .0016); between-group differences also varied across trials from −3.4 mm Hg (95% CI, −5.6 to −1.3 mm Hg) in STEP 2 to −5.1 mm Hg (95% CI, −6.3 to −3.9 mm Hg) in STEP 1.17,18,22,23
Additionally, STEP 2 included the change in glycated hemoglobin (HbA1c) level from baseline to EOT as a confirmatory secondary end point; it showed that patients treated with semaglutide 2.4 mg experienced significantly greater reductions in HbA1c levels compared with patients given placebo. The HbA1c percentage change difference between groups was −1.2% (95% CI, −1.4%; P < .0001).16,22
These results suggest that semaglutide 2.4 mg provides improvements in key measures of metabolic risk by lowering blood pressure and reducing mean waist circumference.17 Additionally, semaglutide 2.4 mg also supports HbA1c improvement in patients with T2D and obesity.22
Physical Function Outcomes
STEPs 1 to 4 included patient-reported outcomes as confirmatory secondary end points; these were assessed using the Short Form 36 v2 Health Survey, Acute Version (SF-36v2). Patients treated with semaglutide 2.4 mg reported improvements in measures of physical functioning on the SF-36v2 from baseline to EOT compared with placebo-treated patients. The greater change in SF-36v2 physical functioning score between groups was significant in STEPS 1, 2, and 4 (STEP 2, P = .0061; STEP 1 and 4, P < .001). However, this change was not significant in STEP 3 (P = .12).17,18,22,23
STEPs 1 and 2 also included a confirmatory secondary end point of change in physical functioning score on the Impact of Weight on Quality of Life-Lite Clinical Trials Version (IWQOL-Lite-CT) measure. By this measure, physical functioning improved significantly more for patients treated with semaglutide 2.4 mg than it did for patients treated with placebo (STEP 1, P < .001; STEP 2, P = .0018).17,22
Overall, treatment with semaglutide 2.4 mg was associated with improvements in physical functioning and quality of life.17,18,22,23
Safety and Tolerability of Semaglutide 2.4 mg in STEPs 1 to 4
Safety results reported across STEPs 1 to 4 are shown in Table 3.16-18,22,23 Overall, the safety profile of semaglutide 2.4 mg was consistent with previous reports for use of GLP-1RAs in clinical trials and with known effects of rapid weight loss; no new safety concerns emerged in analyses of safety data from STEPs 1 to 4.17,18,22,23 Investigators used the Medical Dictionary for Regulatory Activities to identify additional safety areas of interest or focus based on regulatory feedback and requirements and the therapeutic experience previously reported with GLP-1RA treatments.17,18,22,23
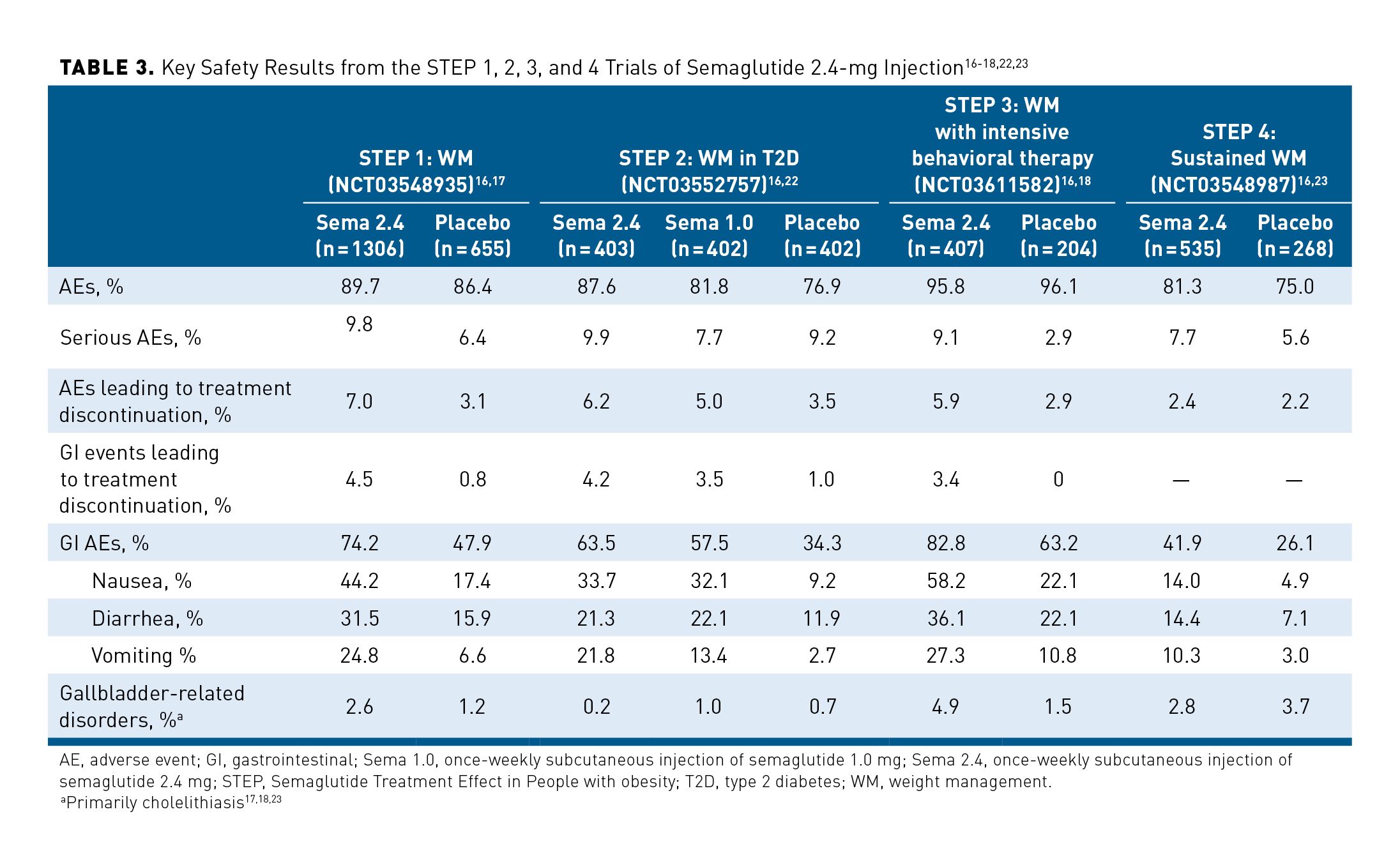
Results of STEPs 1, 3, and 4 demonstrated the safety of semaglutide 2.4 mg when used in patients without T2D. Similar rates of adverse events (AEs) were reported in the semaglutide 2.4-mg and placebo arms across the 68-week STEP 1 and 3 trials.17,18 In STEP 4, a greater percentage of patients who continued use of semaglutide 2.4 mg after 20 weeks reported AEs compared with those who switched to placebo (81.3% and 75.0%, respectively).23 In the STEP 2 trial, which included patients with T2D, a larger percentage of patients reported AEs in the semaglutide 2.4-mg group (87.6%) compared with the semaglutide 1.0-mg (81.8%) or placebo (76.9%) groups.22
Gastrointestinal (GI) disorders were the most frequently reported AEs across STEPs 1 to 4. In STEP 3, which reported the highest rates of GI AEs, 82.8% of patients treated with semaglutide 2.4 mg reported GI AEs vs 63.2% in the placebo arm. Across the trials, nausea, diarrhea, vomiting, and constipation were the most common GI AEs noted, and they occurred more frequently in patients receiving semaglutide 2.4 mg than in patients receiving placebo or semaglutide 1.0 mg. In the semaglutide arm of STEP 3, nausea was reported in 58.2% of patients (vs 22.1% in placebo arm), diarrhea in 36.1% (vs 22.1%), and vomiting in 27.3% (vs 10.8%). In the trials, GI AEs were generally mild to moderate in severity; for the most part, they did not result in treatment discontinuation.The highest rate of discontinuation due to GI AEs (4.5%) was seen in the semaglutide arm of STEP 1.17,18,22,23
Throughout STEPs 1 to 4, serious AEs were reported more frequently with semaglutide 2.4 mg than with semaglutide 1.0 mg or placebo. These were seen in up to 9.8% of patients receiving semaglutide treatment (STEP 1). Treatment discontinuations related to AEs were mostly due to GI disorders; they occurred at a higher incidence in the semaglutide 2.4-mg groups than in the semaglutide 1.0-mg and placebo groups. The highest rate of these discontinuations was seen in STEP 1—7.0% of the semaglutide arm discontinued due to AEs. One death was reported in each of the study groups in STEPs 1, 2, and 4 (placebo, semaglutide 2.4 mg, and semaglutide 1.0 mg [STEP 2]). No deaths were reported in the STEP 3 trial.17,18,22,23
American Gastroenterological Association Recommendation of Semaglutide 2.4-mg Injection
In November 2022, the American Gastroenterological Association (AGA) issued a new clinical practice guideline on pharmacological interventions for adults with obesity.34 Responding to growing evidence from RCTs about AOMs, to the increasing prevalence of obesity and associated conditions, and to the need for guidance on currently available AOMs, guideline authors advanced the evidence-based recommendations of leading societies, including those recommendations discussed by Marc-André Cornier, MD, in the third article of this supplement.34,35
Citing the results of STEPs 1 to 3, STEPs 6 and 8, and other RCTs studying the effects of this agent, AGA guideline authors recommended that semaglutide 2.4 mg “be prioritized over other approved AOMs for the long-term treatment of obesity for most patients.”34
Conclusions
Across STEPs 1 to 4 among adults with obesity with or without T2D, once-weekly subcutaneous semaglutide 2.4 mg demonstrated superiority to placebo and (in STEP 2) to once-weekly subcutaneous semaglutide 1.0 mg as an adjunct to lifestyle modification with respect to weight loss. Semaglutide 2.4 mg provided substantial weight loss that was both clinically and statistically significant, and its safety and tolerability profiles appeared to be similar to those of other GLP-1RAs.
In STEP 1, adults with obesity and without T2D who received semaglutide 2.4 mg had a mean weight loss of 14.9%, which exceeded placebo-based therapy by 12.4%.17 Although semaglutide 2.4 mg was not investigated in head-to-head trials with other AOMs, the 14.9% reduction seen in STEP 1 was considerably larger than the 4.0% to 10.9% reductions in body weight seen across phase 3 development programs of other agents.7 Moreover, 86% of the semaglutide treatment group of this trial lost 5% or more of their baseline body weight, which is the minimum recommended loss to mitigate certain weight-related complications.2,17 Importantly, as compared with patients treated with placebo, those treated with semaglutide also were significantly more likely to lose the 10% to 15% of baseline body weight that is recommended to mitigate many weight-related complications.2 Semaglutide-treated patients were 14.7 times more likely to lose at least 10% of their baseline body weight and 19.3 times more likely to lose at least 15% of their body weight than were placebo-treated patients.17
In STEP 1, waist circumference and blood pressure also were reduced to a significantly greater degree for patients receiving semaglutide than they were for those receiving placebo. The semaglutide treatment group lost a mean 9.4 cm more in waist circumference and dropped 5.1 mm Hg more in blood pressure than did the placebo group.17 Finally, as compared to treatment with placebo, treatment with semaglutide was associated with improved physical functioning and quality of life—the mean SF-36v2 and IWQOL-Lite-CT physical functioning scores for the semaglutide treatment group were higher than they were for the placebo group.17
Results of STEPs 2 to 4 corroborated the efficacy and safety seen in STEP 1, across patients with T2D (STEP 2) and patients also receiving intensive behavioral therapy (STEP 3), and in comparison to patients who were withdrawn from semaglutide therapy at 20 weeks (STEP 4). Results of STEP 2 also demonstrated that patients treated with semaglutide 2.4 mg had significantly greater reductions in HbA1c levels than did patients given placebo and significantly greater weight loss than did patients given semaglutide 1.0 mg or placebo.22
The FDA approval of once-weekly subcutaneous semaglutide 2.4 mg was based upon the results of the STEP 1 to 4 trials, which demonstrate that semaglutide 2.4-mg injection is a safe, well tolerated, and highly effective treatment to promote weight loss, avoid weight regain, and reduce risk factors associated with obesity. As shown in Table 1, the full STEP program seeks to demonstrate the degree to which semaglutide 2.4 mg SC is associated with weight loss recommended to ameliorate comorbidities, as well as its safety and efficacy relative to other GLP-1RAs and other AOMs.16-18,20-33 Based on results from the STEP trials and other RCTs of this agent, the AGA recommends that semaglutide 2.4-mg injection be prioritized for the long-term treatment of obesity for most patients.34
Acknowledgements
This peer-reviewed supplement was funded by Novo Nordisk Inc. The authors acknowledge the professional medical writing support from Clinical Communications, a division of MJH Life Sciences®, Cranbury, NJ, which received funding support from Novo Nordisk Inc, Plainsboro, NJ. Novo Nordisk Inc. provided scientific and medical accuracy review of this publication.
Author Affiliations: Jefferson Health and Sidney Kimmel Medical College at Thomas Jefferson University (JK), Philadelphia, PA.
Funding Source: This supplement was supported by Novo Nordisk.
Author Disclosures: Dr Kyrillos reports serving as a paid advisory board member for Lilly on the US Medical Education Obesity Advisory Board. She also reports receiving lecture fees from Novo Nordisk: Speaker’s Bureau.
Authorship Information: Concept and design (JK); drafting of the manuscript (JK); and critical revision of the manuscript for important intellectual content (JK).
Address Correspondence to: Janine Kyrillos, MD, 225 East City Ave, Bala Cynwyd, PA 19004. Email: janine.kyrillos@jefferson.edu
References
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8. CDC; National Center for Health Statistics. Reviewed February 27, 2020. Accessed October 21, 2022. https://www.cdc.gov/nchs/products/databriefs/db360.htm
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi:10.4158/EP161365.GL
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25; part B):2985-3023. doi:10.1016/j.jacc.2013.11.004
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415.
- Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175-247. doi:10.1016/j.soard.2019.10.025
- Shetye B, Hamilton FR, Bays HE. Bariatric surgery, gastrointestinal hormones, and the microbiome: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;2(1):1-20. doi:10.1016/j.obpill.2022.100015
- Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237-248. doi:10.1016/S2213-8587(17)30236-X
- Bydureon. Prescribing information. Amylin; 2012. FDA. Accessed July 25, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022200s000lbl.pdf
- Trulicity. Prescribing Information. Eli Lilly & Company; 2022. Accessed July 25, 2022. https://pi.lilly.com/us/trulicity-uspi.pdf
- Victoza®. Prescribing Information. Novo Nordisk; 2022. Accessed July 25, 2022. https://www.novo-pi.com/victoza.pdf
- Saxenda®. Prescribing Information. Novo Nordisk; 2022. Accessed July 25, 2022. https://www.novo-pi.com/saxenda.pdf
- Wegovy®. Prescribing Information. Novo Nordisk; 2021. Accessed July 25, 2022. https://www.novo-pi.com/wegovy.pdf
- Ozempic®. Prescribing Information. Novo Nordisk; 2022. Accessed July 25, 2022. https://www.novo-pi.com/ozempic.pdf
- Rybelsus®. Prescribing Information. Novo Nordisk; 2022. Accessed July 25, 2022. https://www.novo-pi.com/rybelsus.pdf
- O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637-649. doi:10.1016/S0140-6736(18)31773-2
- Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050-1061. doi:10.1002/oby.22794
- Wilding JPH, Batterham RL, Calanna S, et al; STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Wadden TA, Bailey TS, Billings LK, et al; STEP 3 Investigators. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi:10.1001/jama.2021.1831
- FDA approves new drug treatment for chronic weight management, first since 2014. FDA. June 4, 2021. Accessed July 25, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- STEP 1: research study investigating how well semaglutide works in people suffering from overweight or obesity (STEP 1). ClinicalTrials.gov. Updated November 19, 2021. Accessed July 25, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03548935
- Wilding JPH, Batterham RL, Davies M, et al; STEP 1 Study Group. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553-1564. doi:10.1111/dom.14725
- Davies M, Færch L, Jeppesen OK, et al ; STEP 2 Study Group. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomized, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi:10.1016/S0140-6736(21)00213-0
- Rubino D, Abrahamsson N, Davies M, et al; STEP 4 Investigators. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-1425. doi:10.1001/jama.2021.3224
- Two-year research study investigating how well semaglutide works in people suffering from overweight or obesity (STEP 5). ClinicalTrials.gov. Updated March 23, 2021. Accessed July 25, 2022. https://clinicaltrials.gov/ct2/show/NCT03693430
- STEP 6: research study investigating how well semaglutide works in people living with overweight or obesity (STEP 6). ClinicalTrials.gov. Updated March 22, 2022. Accessed July 25, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03811574
- Research study of how well semaglutide works in people living with overweight or obesity (STEP 7). ClinicalTrials.gov. Updated March 31, 2022. Accessed July 25, 2022. https://www.clinicaltrials.gov/ct2/show/NCT04251156
- Research study to investigate how well semaglutide works compared to liraglutide in people living with overweight or obesity (STEP 8). ClinicalTrials.gov. Updated April 27, 2022. Accessed July 25, 2022. https://www.clinicaltrials.gov/ct2/show/NCT04074161
- Effect of subcutaneous semaglutide 2.4 mg once-weekly compared to placebo in subjects with obesity and knee osteoarthritis. EudraCT Number: 2020-000204-11. EU Clinical Trials Register. Accessed July 25, 2022. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000204-11/SE
- Research study looking at how well semaglutide works in people suffering from obesity and knee osteoarthritis. ClinicalTrials.gov. Updated June 22, 2022. Accessed July 25, 2022. https://clinicaltrials.gov/ct2/show/NCT05064735
- Kadowaki T, Isendahl J, Khalid U, et al; STEP 6 Investigators. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomized, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193-206. doi:10.1016/S2213-8587(22)00008-0
- Research study looking at how well semaglutide works in people living with obesity and prediabetes (STEP 10). ClinicalTrials.gov. Updated March 23, 2022. Accessed July 25, 2022. https://clinicaltrials.gov/ct2/show/NCT05040971
- Research study to investigate how well semaglutide works in people living with heart failure and obesity (STEP-HFpEF). ClinicalTrials.gov. Updated June 16, 2022. Accessed July 25, 2022. https://clinicaltrials.gov/ct2/show/NCT04788511
- Research study to look at how well semaglutide works in people living with heart failure, obesity and type 2 diabetes (STEP HFpEF DM). ClinicalTrials.gov. Updated March 21, 2022. Accessed August 17, 2022. https://www.clinicaltrials.gov/ct2/show/NCT04916470
- Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022;163(5):1198-1225. doi:10.1053/j.gastro.2022.08.045
- Cornier MA. A review of current guidelines for the treatment of obesity. Am J Manag Care. 2022;28(suppl 15):S288-S296. doi:10.37765/ajmc.2022.8929

