- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Risankizumab Found Effective in Patients With Moderate Plaque Psoriasis
A new analysis highlights the effectiveness of risankizumab in patients with moderate plaque psoriasis and those newly classified as systemic therapy eligible under International Psoriasis Council guidelines.
Despite traditionally being excluded from systemic therapy trials, patients with moderate psoriasis—defined as having Body Surface Area (BSA) of 3% to 10% or involvement of high-impact areas—are now recognized as eligible under the International Psoriasis Council’s (IPF’s) updated classification.1 A study of real-world data demonstrated that 12 months of risankizumab treatment led to substantial skin clearance and quality of life improvements in this previously underrepresented group.
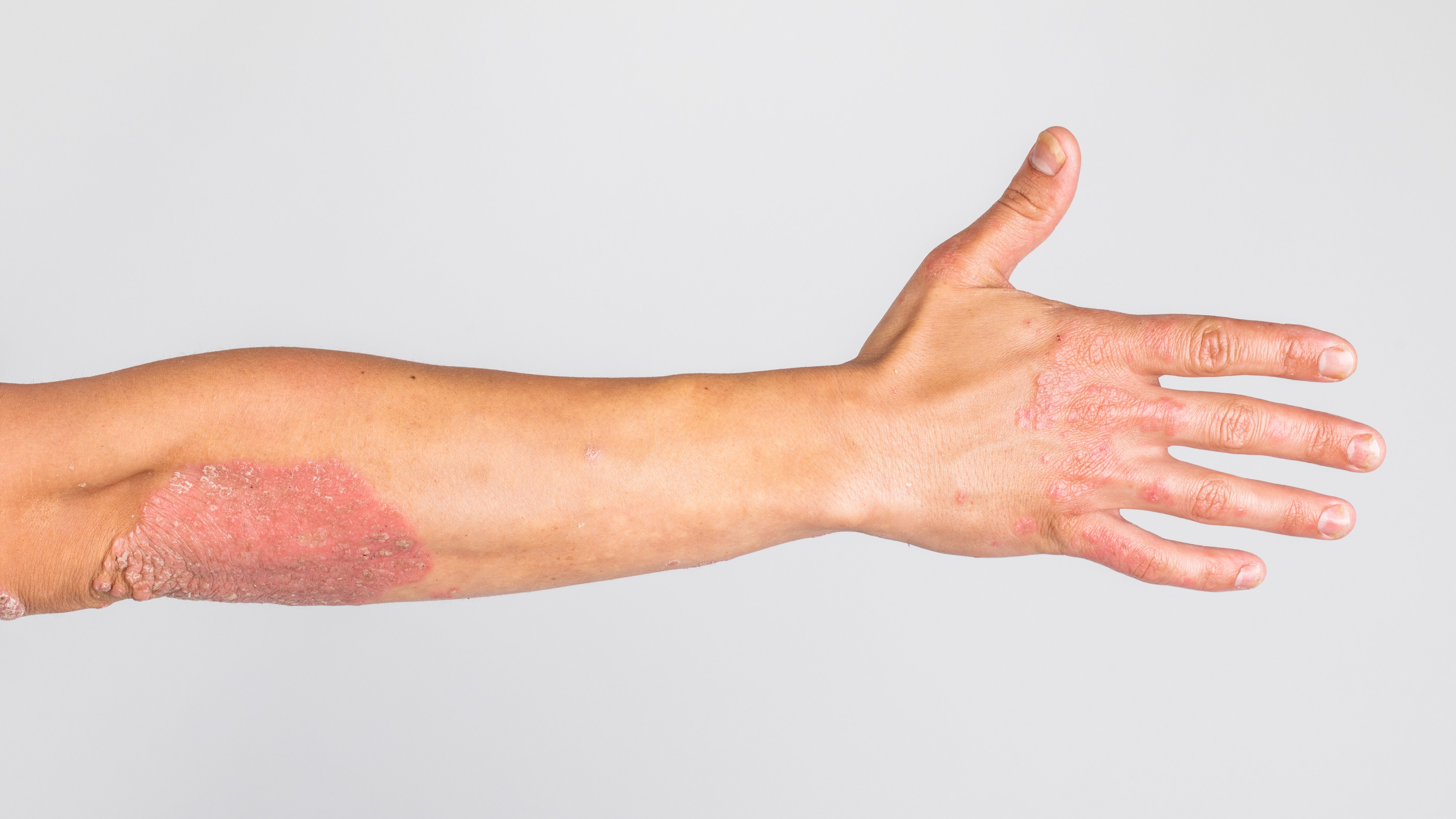
This retrospective study is published in Dermatology and Therapy.
“After 12 months of continuous treatment with risankizumab, all patient groups evaluated in this study showed significant improvements in their PASI [Psoriasis Area Severity Index] scores,” wrote the researchers of the study. “The majority achieved NPF [National Psoriasis Foundation] treat-to-target goals and had significant improvement in quality of life, psoriasis symptoms, and reduced work and activity impairment.”
Although psoriasis treatment guidelines have historically focused on patients with BSA involvement greater than 10%, growing evidence highlights that individuals with BSA of 3% or less can experience a disease burden comparable to those with more extensive skin involvement.2 Data from the CorEvitas Psoriasis Registry show that patient-reported outcomes (PROs) related to itch, pain, fatigue, and quality of life significantly overlap across low, medium, and high BSA categories.
This analysis utilized data from the CorEvitas Psoriasis Registry to evaluate real-world outcomes in biologic-naïve adults with moderate to severe plaque psoriasis who initiated risankizumab treatment between April 2019 and August 2023.1 Eligible patients received continuous risankizumab therapy for 12 months and were stratified by baseline BSA: 3% to 10% or greater than 10%.
Within the BSA 3% to 10% subgroup, patients were further assessed for involvement of high-impact areas and prior use of topical therapy, aligning with the IPF criteria for systemic therapy eligibility. Effectiveness outcomes included achievement of Psoriasis Area and Severity Index (PASI) 90 and 100 and National Psoriasis Foundation (NPF) treat-to-target goals. PROs included the proportion achieving a Dermatology Life Quality Index (DLQI) score of 0/1, as well as changes in symptom burden and work or activity impairment from baseline.
Among the 272 patients included in the analysis, 123 had baseline BSA between 3% and 10%, of whom 78 had psoriasis in high-impact areas and 105 had a history of topical therapy use, meeting IPC criteria for systemic eligibility. The remaining 149 patients had BSA greater than 10%. In the BSA 3% to 10% group, 77.9% achieved PASI 90 and 67.2% achieved PASI 100 after 12 months of risankizumab treatment. Additionally, 95.3% met the NPF’s acceptable target, and 87.9% reached the optimal target.
PROs were similarly encouraging, with 68.1% achieving a DLQI score of 0/1, indicating minimal impact on quality of life. Statistically significant improvements were also observed in psoriasis symptom burden and reductions in both work and activity impairment (P < .001). Furthermore, comparable clinical and quality of life benefits were seen across all IPC-defined subgroups, supporting the effectiveness of risankizumab in patients beyond traditional systemic therapy thresholds.
However, the researchers noted several limitations. Although safety outcomes were not evaluated, prior clinical trials have demonstrated a favorable long-term safety profile for risankizumab. Ongoing studies (EUPAS3935; NCT04799990) aim to assess the real-world safety of risankizumab more comprehensively. Additionally, the current analysis was limited by the absence of head-to-head comparisons with other biologic or systemic therapies in patients newly classified as systemic-eligible under IPC guidelines. The lack of comparable real-world studies for alternative treatments also limited these findings.
Despite these limitations, the researchers believe the study provides evidence that supports risankizumab in patients eligible for systemic therapy under IPC guidelines.
“Given these outcomes, risankizumab may be considered a primary systemic therapy option for treating patients eligible for systemic therapy,” wrote the researchers.
References
1. Strober B, Patel M, Kaldas MI, et al. Real-world skin clearance and quality of life with risankizumab in patients with psoriasis with moderate skin involvement and those eligible for systemic therapy per International Psoriasis Council classification. Dermatol Ther (Heidelb). Published online July 2, 2025. doi:10.1007/s13555-025-01474-3
2. Steinzor P. Low BSA in psoriasis may still mean high disease burden. AJMC®. June 12, 2025. Accessed July 2, 2025. https://www.ajmc.com/view/low-bsa-in-psoriasis-may-still-mean-high-disease-burden
Psoriasis as an Inflammatory Disease, and What’s Changed Over Time
August 3rd 2021August is National Psoriasis Awareness Month, and on this episode of Managed Care Cast, we bring you an excerpt of an interview with a New Jersey dermatologist about the changing concept of psoriasis as more than just a skin disease.
Listen
