- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Rethinking Heart Failure: Patient Classification and Treatment
ABSTRACT
INTRODUCTION: Approaches to treating heart failure (HF), understanding of the most timely and effective interventions, and identification of appropriate patient subpopulations must evolve. HF has emerged as a chronic condition that needs to be managed on multiple fronts. Hospital resources are more limited than ever due to various factors that directly impact staff and hospital space available to manage and treat patients with HF. As a result, there is increasing attention to the current state of this progressive disease and ways to improve patient outcomes.
PURPOSE: This paper examines HF and the current and future treatment landscape, the need to reevaluate terms and definitions, and the opportunity to treat HF with the right treatment at the right time. Treatments in development and potential new investigational therapies are also discussed.
CONCLUSION: To meet the current challenge, HF treatment must adapt. For other disease states, we have more personalized, nimble, and timely treatment strategies that harness windows of opportunity to help maximize outcomes and reduce overwhelming costs to the health care system. HF treatment is evolving with new guidelines and treatments that hold the promise of greater personalization through additions to existing treatments that are directed by medical guidelines, since each patient is unique and requires more than a one-size-fits-all approach. In addition, advances in remote monitoring, in-home care, and telemedicine are creating a more individualized treatment approach. Therefore, it becomes critical for all health care decision makers to be aware of the tools and resources available in treatment guidelines, individualized treatment options, telemedicine, and other ways of expanding the existing toolbox to enhance patient centricity in HF treatment.
Am J Manag Care. 2022;28(suppl 14):S255-S267. https://doi.org/10.37765/ajmc.2022.89272
For author information and disclosures, see end of text.

Cardiovascular disease (CVD) is the leading cause of death in the world, with an estimated 17.9 million fatalities in 2019 that represented 32% of all deaths. Of these deaths, a staggering 85% were due to heart attack and stroke.1 Heart failure (HF) is a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood. HF often affects only the left or right side of the heart, but it can affect both. Left-sided HF is usually caused by coronary artery disease, a heart attack, or long-term uncontrolled high blood pressure.1 Right-sided HF generally results from advanced left-sided HF or significant pulmonary disease. Biventricular HF affects both sides of the heart, and it can cause the same symptoms as left-sided and right-sided HF. The type of HF that patients have upon presentation determines the medication they will use for treatment.1 This paper focuses on the most predominant type of HF—left-sided HF—which will be referred to as “HF” throughout.
The incidence of HF has reached epidemic proportions; it is the most rapidly growing CVD health burden worldwide.2 Absolute numbers of patients with HF have been increasing due to the aging population, global population growth, and improved survival after diagnosis.3 With an estimated 64.3 million individuals living with HF globally, it is a clinical and public health crisis associated with significant mortality, morbidity, and health care expenditures, particularly among those 65 years and older (Text Box).3-11


Kaiser Permanente researchers reported an epidemic of increasing deaths due to HF in the United States, particularly among individuals 65 years and older (Figure 1).12-15
Over 647,000 individuals in the United States died of heart disease in 2017—about 51,000 more than in 2011. HF was the underlying cause of 80,000 deaths in 2017—about 22,000 more than in 2011.12
Moving forward, it will become more critical to focus on heart health for those aged 65 years and older, which grew by approximately 10 million individuals between 2011 and 2017 and is projected to increase by another 22 million by 2030.12

HF Is the Leading Cause of Hospitalization Among Older Adults8
Until 2012, the rate of hospitalizations for HF in the United States was decreasing; however, from 2013 to 2017, the trend reversed. In 2017, there were 1.2 million HF hospitalizations in the United States among 924,000 patients with HF, representing a 26% increase from 2013 in HF hospitalizations and the number of patients hospitalized with HF. The increase in patient hospitalizations is largely due to the aging population.16
Hospitalization costs are the key driver of HF-related costs. The total cost of HF care in the United States exceeds $30 billion annually.5 Approximately three-fourths of patients with primary or comorbid HF who had emergency department (ED) visits and hospitalizations were Medicare beneficiaries; further, Medicare beneficiaries with HF have the highest readmission rate of patients with any condition.8
Understanding the Evolution of HF
Ejection fraction (EF) has emerged as a unique predictor of CV outcomes. It is a measurement expressed as the percentage of blood the left ventricle (LV) pumps out with each contraction. Reduced EF is key to a patient’s disease progression, because a decrease in cardiac output triggers a pathologic chain of events that results in systolic HF. In fact, in patients with HF, declining LVEF is a meaningful and strong predictor of CV outcomes, including all-cause mortality, CV mortality, sudden death, HF-related death, fatal or nonfatal myocardial infarction (MI), and HF hospitalization.17
HF, in general, is often described as a complex condition with a wide range of manifestations; its categorization has evolved to comprise 4 subtypes: HF with reduced EF (HFrEF), HF with improved EF (HFimpEF), HF with mildly reduced EF (HFmrEF), and HF with preserved EF (HFpEF) (Figure 2).16 These subtypes differ considerably in terms of demographic distribution, underlying pathophysiologic mechanisms, and available preventive and therapeutic options.16,18

Characterized by an EF at or below 40%, HFrEF is accompanied by progressive LV dilatation and adverse cardiac remodeling (Figure 3).9,17-22 It is often preceded by acute or chronic loss of cardiomyocytes due to ischemia, infarction, myocarditis, genetic mutation, or valvular disease.2
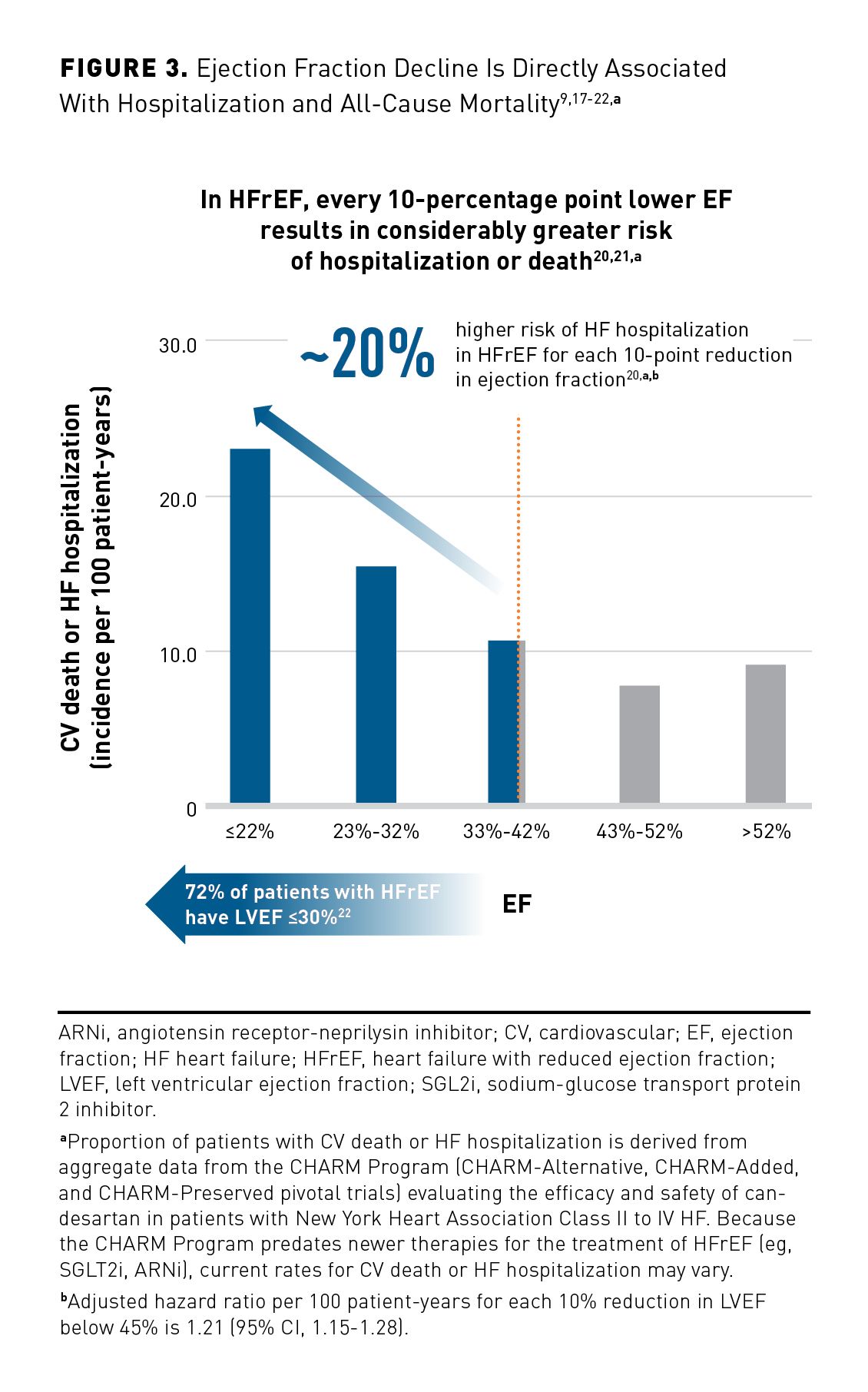
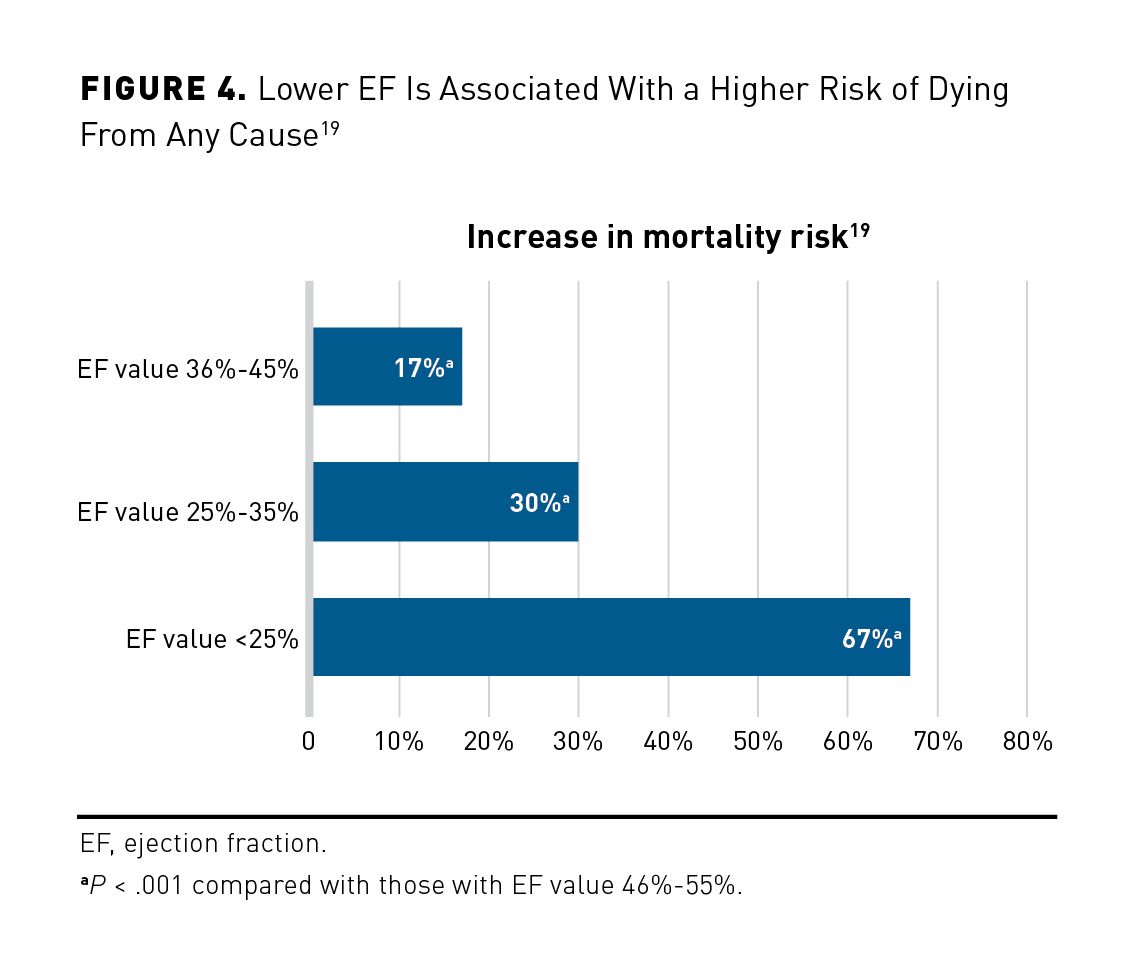
A recent retrospective cohort study followed 27,323 patients and 19,445 matched controls for over 223,000 patient-years while measuring outcomes based on EF values.19 Regardless of CVD history, EF significantly predicted the risk of several different outcomes, including total mortality, CV death, CV hospitalizations, and HF hospitalizations (Figure 4).19
Redefining What It Means to Have HF
The New York Heart Association (NYHA) and the American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) are the medical societies that developed systems for classifying and staging HF. The NYHA and the ACC/AHA/HFSA have created 2 systems that are complementary.16
The NYHA classification is a subjective assessment used to characterize symptoms and functional capacity of patients with symptomatic (stage C) HF or advanced HF (stage D). It is a subjective assessment by a clinician that can change over time; however, it is widely used in clinical practice to determine the eligibility of patients for treatment strategies.16
The ACC/AHA/HFSA guidelines provide an evidence-based approach to managing HF with the intent to improve patient quality of care. The guidelines characterize HF disease progression using 4 stages; it recently underwent significant modification—including a revised definition for the condition. The changes represent a shift in perspective and a building momentum toward significant change in HF treatment. The ACC/AHA/HFSA recognized the need to provide more patient-centric, evidence-based recommendations for clinicians (Figure 5).16
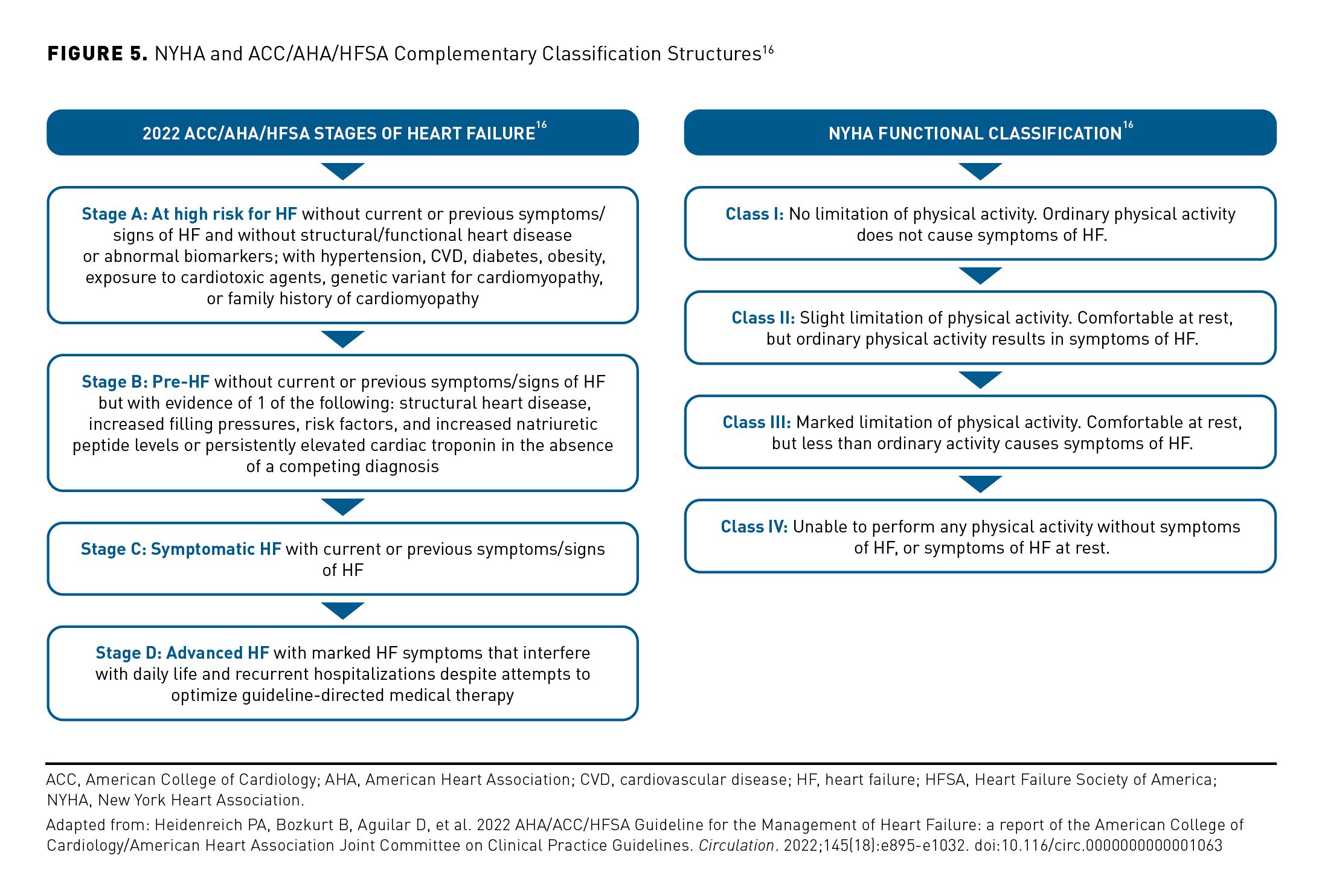
The guidelines are evolving and bringing more attention and emphasis to HF subtypes. For example, updates include a new category of EF, HF with improved ejection fraction, or HFimpEF. In these patients, treatment should be continued to prevent relapse of HF and LV dysfunction, even in patients who may become asymptomatic, since symptom resolution and improvement in cardiac function after treatment should be classified as remission, not full and sustained recovery. According to the new guidance, patients with previous HFrEF who have an EF over 40% are now classified as having “improved EF” and should continue HFrEF treatment.16 The evolution of the guidelines increasingly reflects HF as a disease without a static trajectory. There are windows of opportunity to change course and adjust treatment accordingly for a chronic condition.
Another critical adaptation to the ACC/AHA/HFSA guidelines is the identification of early risk factors for HF (stage A) and patients at risk of more severe disease. This takes a more preventive approach to help provide treatment before structural changes or signs of decreased heart function occur (stage B). Stage A is now defined as “at risk for HF,” stage B as “pre-HF,” stage C as “symptomatic HF,” and stage D as “advanced HF.” The revisions also introduce a trajectory of stage C HF that recognizes that symptoms of patients with HFrEF may improve or worsen, yet the patients remain in stage C and require continued treatment or modifications.16
The 2022 ACC/AHA/HFSA guidelines emphasize prevention and individualized treatment and define HF as a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood.16
Overall, the updated definitions, classifications, and stages of HF are steps to achieve clarity and uniformity of care. However, a rather low percentage of patients are treated by all indicated therapies, and the majority of these patients do not reach target doses of the guideline-recommended treatments.23 Consequently, much work needs to be done to reverse treatment inertia that resulted from many contributing factors.23 The NYHA functional classification system can have multiple interpretations, which is a limitation of symptom-driven treatment strategies in HF.24
Changes Are Occurring on a Global Scale
Due to the need for universal consensus in the definitions and classifications of HF, members of the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society assembled a consensus document emphasizing that HF is a clinical syndrome with symptoms and/or signs caused by structural and/or functional cardiac abnormality.20
Realizing that semantic changes are needed, the committee also targeted a universal definition and classification of HF:
- There is a need to move away from the traditionally accepted term “heart failure” and transition to the term “heart function.”25
- Clinicians should opt for the term “persistent HF” rather than “stable HF,” because, even during stable HF, there are opportunities to optimize therapies that prevent further worsening and/or deterioration or adverse outcomes.5
- “HF in remission” is defined as an important substitution for the conventionally used term “recovered HF” for patients who experience resolution of their symptoms and/or systolic function, as HF is known to frequently relapse. It is important that clinicians convey to patients that improvement does not mean that their HF is cured.5
Impact of Social Determinants of Health on HF Management
Approximately 3.6 million women in the United States are affected by HF, and their experience is different than that of men. The causes of HF in women also are different; they are linked to high blood pressure, coronary artery disease, valvular disease, and diabetes.23 These causes may explain why women with HF require hospitalization more frequently than men, with approximately 40% of patients hospitalized with HFrEF being women.22 After women develop coronary heart disease, the risk of HF is high.26
Black patients are underprescribed certain treatments. As with women, treatment modalities are different for Black patients.27 For example, hydralazine-isosorbide dinitrate (H-ISDN) therapy is recommended to treat those with moderate to severe HFrEF.16 Despite guideline recommendations, very few contemporary studies have examined the real-world use of H-ISDN. A 2017 study of 5168 Black patients with HF between 2007 and 2013 found that H-ISDN remained underused in those with HF and HFrEF.28 In addition, Black patients may need treatment adjustments based on use of concomitant therapies and differing treatment modalities compared with other patient populations.28
Despite these disparities, current evidence of HF treatment is limited to clinical trials, not real-world, patient populations.26 Therapeutic developments in HF apply mostly to men; they have not been adequately studied in women. Similarly, Black patients have been underrepresented in clinical trials, and studies have not been designed to prospectively study differences in treatment outcomes. As a result, only approximations of risks and benefits can guide therapy for the least-studied populations.29
New research is shedding light on social determinant disparities in clinical terms. For example, the concept of lifetime risk is now considered to be important, and this has shed light on population differences in HFrEF risk. Lifetime risk estimates account for the chance of developing the disease of interest and the risk of competing causes of death. An analysis confirmed that lifetime risks for HFrEF vary by sex, race, and history of antecedent MI.18 Among participants with antecedent MI before HF diagnosis, lifetime risks for HFrEF increased 4-fold compared with those without antecedent MI.
Guideline-Directed Medical Therapy and Individualized Strategies
The use of guideline-directed medical therapy (GDMT) reduces morbidity and mortality in HF.30 However, despite a few recent breakthroughs in HF treatment, the current standard of care is falling short. Clinicians and payers are eagerly awaiting HF treatments that can improve symptoms and reduce both admissions and mortality without deleterious effects. Changes and classification schemes in the new definition offer opportunities for better communication and patient engagement, wherein clinicians can focus on implementation of GDMT across a continuum to improve outcomes.
GDMT refers to initial medical therapy with an angiotensin receptor-neprilysin inhibitor (ARNi), a β-blocker, a sodium-glucose transport protein 2 (SGLT2) inhibitor, and a mineralocorticoid receptor antagonist.31 Patients who do not have access to an ARNi (often for financial reasons) should receive an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). As evidenced by the increase in morbidity and mortality in patients with HFrEF, optimized GDMT may no longer be viable for patients who have exhausted GDMT treatment options. In addition, some of the recommendations may be useful for early stages of HF but lack evidence of efficacy at more advanced stages.
GDMT has the potential to be the mainstay of pharmacologic therapy for HFrEF.16 However, it presents inherent challenges, because patients with HF represent a heterogeneous cohort with multiple comorbidities. Among Medicare beneficiaries with chronic HF, 41% to 55% have 5 or more noncardiac comorbidities, which increases the risk of hospitalization in this patient population and all hospitalizations proportionately.32
A 2020 review of HF clinical trials published between 2001 and 2016 with a cumulative total of 215,508 patients uncovered that the reporting of comorbidities was more common in HFrEF trials. Common comorbidities included hypertension, ischemic heart disease, hyperlipidemia, diabetes, atrial fibrillation, and chronic kidney disease. Conditions with the largest increases over time were hypertension, atrial fibrillation, and chronic kidney disease. In addition, overall rates of comorbidity reporting in HF clinical trials were low, which may be due to criteria excluding certain comorbidities to reduce competing mortality risk and/or to improve tolerability of therapy. Other studies have shown an increase in the number of patients with HF with multiple comorbidities over time, which may explain the recent US rise in HF mortality.33


In the clinical setting, implementation of HF treatment is not consistent with current guidelines (Figure 6).24,34-37
The new ACC/AHA/HFSA guidelines call for better incorporation of and devotion to social health determinants for vulnerable patient populations at risk for health disparities. Multidisciplinary management strategies should target both known risks for CVD and social determinants of health to level the playing field and address disparate outcomes in HF.16 Optimal pharmacologic therapy of HFrEF is meticulously selected based on evidence-based treatment guidelines; however, a large proportion of patients with chronic HFrEF either do not receive GDMT or are underdosed in clinical practice. This may be due to tolerability issues related to low blood pressure, low heart rate, impaired renal function, or hyperkalemia. Adding to the complexity of HF treatment is that most patients usually receive multiple concomitant therapies. Therefore, a personalized approach to HF treatment (eg, adjusting GDMT to patient profiles) may help achieve more accurate and comprehensive therapy for each patient than is available with more traditional, forced titration of each drug class before the next treatment is started. Patients with HFrEF who develop worsening HF may not receive optimal treatment at critical times throughout their HF journey. In addition, novel treatments are needed for patients with worsening HF, especially if they do not tolerate available HF therapies.34
Rethinking Treatment of Worsening HF
In clinical practice, patients with HF are often treated reactively after symptoms arise. In particular, attention has been focused on worsening HF (WHF), which carries an unfavorable prognosis and presents an opportunity for intensification and personalization of treatment for HF. WHF is common and defined as worsening HF signs and symptoms in a patient with chronic HF after a period of clinical stability that requires escalation of therapy.38
WHF, an effective measure in making treatment decisions, allows use of more personalized therapies at the optimal time. This may be especially critical for a patient population for whom time is of the essence and specialized therapies may help sooner.
When presenting with reduced EF, WHF is a major public health concern with substantial morbidity and mortality.38 It is now recognized as worsening or deterioration of HF signs and symptoms in a patient with chronic HF after a period of clinical stability that requires escalation of therapy and is a clinical entity independent of the location of care.37 The choice of location (eg, inpatient, outpatient, ED) is inherently subjective and subject to multiple potential clinical and nonclinical factors.37 Based on recent literature, patients with WHF typically present with any of the following paramaters10:
- NT-proBNP level above 3800 pg/mL, or
- EF of 35% or less, or
- ED visit or hospitalization for HF in the past 12 months.
WHF is increasingly used as an accepted inclusion criterion, end point, or part of a combined end point in clinical trials.10 In general terms, WHF is worsening of chronic HF that occurs during hospitalization or in the ambulatory setting. It often results in adjustments to long-term therapy and inpatient hospitalization and is associated with an unfavorable prognosis (Figure 7).38-41
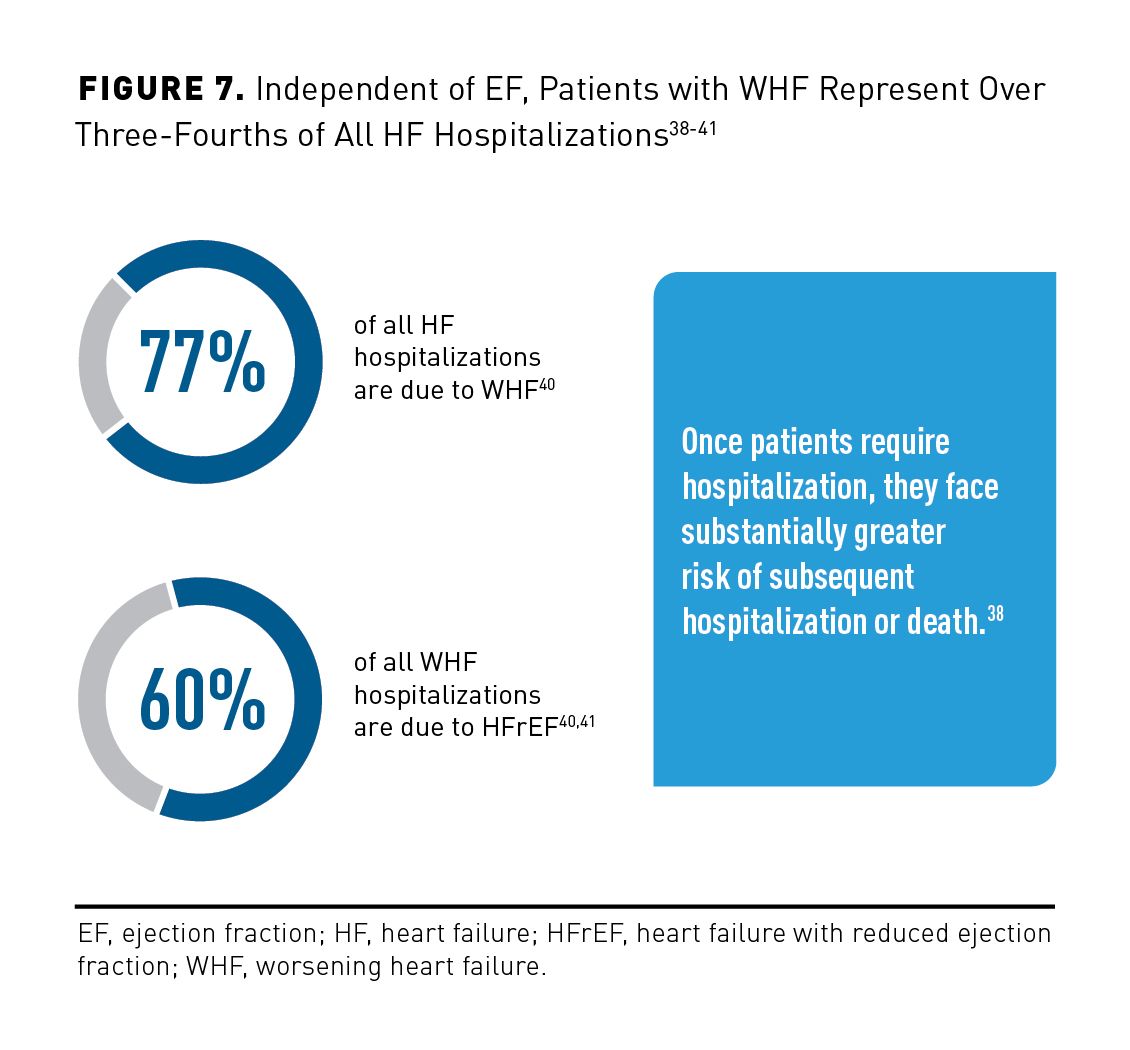

Emerging data suggest that WHF needs to be considered beyond hospitalization. It does not occur only in the hospital, as it is not based on location. Patients may experience WHF in the outpatient setting, and hospitalization or ED presentation may be avoided with adequately adjusted therapy.42 Select comorbidities can result in discontinuation of GDMT for hospitalized patients. For instance, worsening renal failure is common when managing chronic HF, and it may require discontinuation of ACE inhibitors/ARBs because of concerns about hyperkalemia.43 Chronic kidney disease plays an important role, as it affects up to 50% of patients with HF. The strongest predictors of adequate GDMT consist of absence of chronic renal disease or nonsustained ventricular tachycardia, low-income prescription benefits subsidy, and most recent EF evaluation occurring more than 1 month before placement of an implantable cardioverter-defibrillator.44
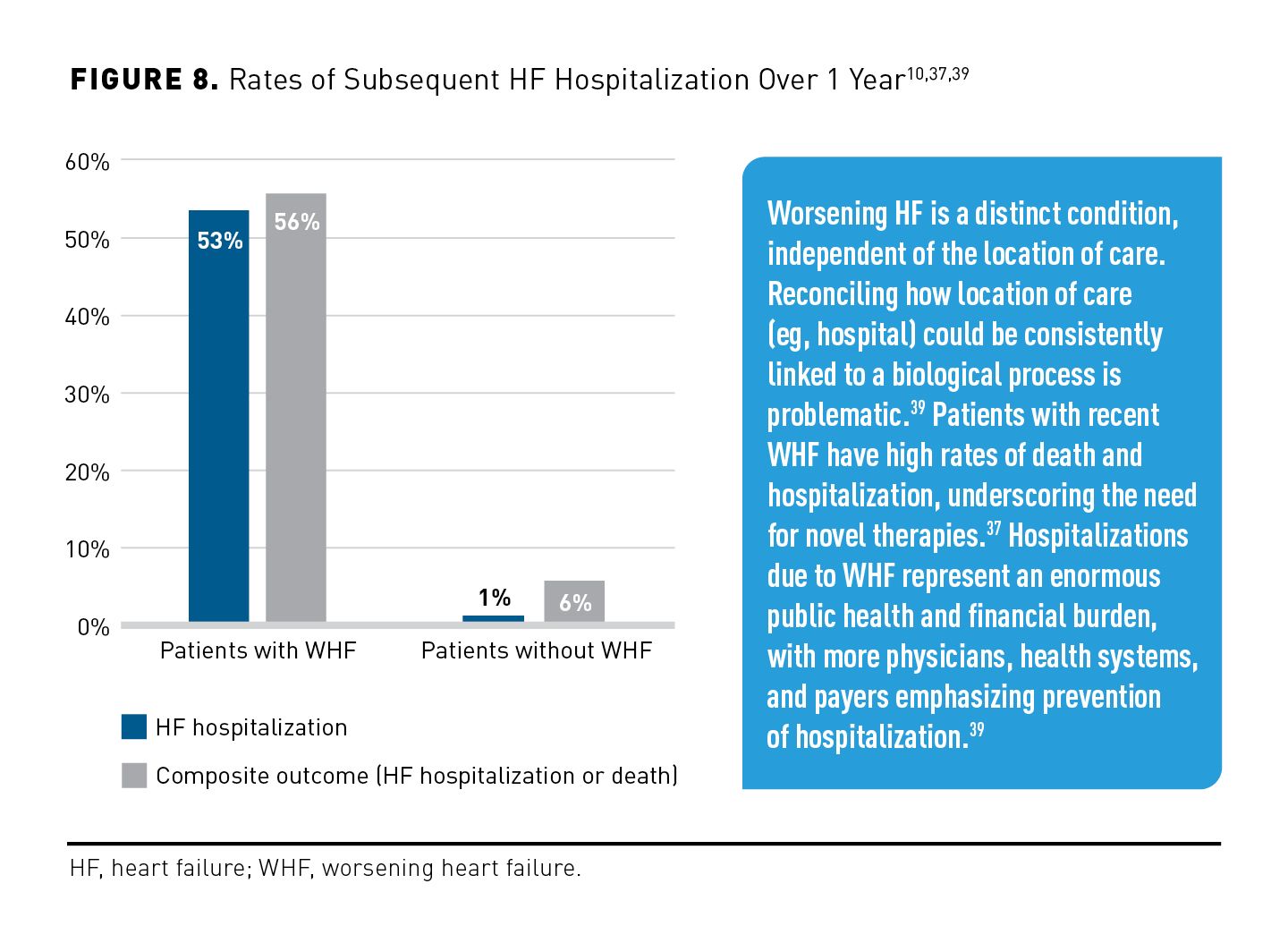
As shown in Figure 8,10,37,39 over a 1-year period, patients with WHF had a significantly higher rate of subsequent HF hospitalization than did those without WHF (53% vs 1%; P < .001) and a significantly higher rate of composite outcome of HF hospitalization or CV death (56% vs 6%; P < .001).10
Effect of Hospitalization on Outcomes in Patients With HF
With high rates of mortality and high risk of readmission, hospitalizations for HF are significant events with downstream implications for patients, health care systems, and payers. Long-term outcomes among acute HF survivors remain poor despite a similar case mix over the past 2 decades. Increased study of management strategies after hospital discharge is imperative, including but not limited to prognosis reassessment, inpatient-to-clinic transitions, and HF systems-based ambulatory care.45
Recurrent hospitalization, morbidity, and mortality as a result of HF remain high despite increasing technology for pharmacotherapy and device-based therapies.46 Patients with a lower EF have a significantly increased risk of hospitalization due to HF compared with patients with the highest EF.19 HFrEF is regarded as the most challenging HF syndrome to treat—with patients in dire need of help and having the highest rates of hospitalization.47-49
The previously mentioned 2020 case-controlled cohort study of 27,323 patient outcomes that included total mortality, CV death, CV hospitalizations, and HF hospitalizations as a function of EF found that19:
- Hospitalization rates among patients with EF below the 36% to 45% range were more than double those of other EF groups,
- HF hospitalizations were 2- to 3-fold higher among those with an EF less than 25% compared with the 46% to 55% group, and
- Overall, the highest number of hospitalizations was observed among patients with an EF from 25% to 35%.
Rehospitalization Rates Are Not Only Increasing—They Are Accelerating
Rehospitalization is a major issue for patients with HF, as it could increase the risk of death, result in poor quality of life (QOL), and become an enormous economic burden.50 Rehospitalization rates for HF are among the highest across diagnoses. Approximately 20% of patients with HF are readmitted within 30 days, and about 50% are readmitted within 6 months after leaving the hospital (Figure 9).51 There is a dire need for vigilance and urgency following discharge due to progressively shortened intervals from HF discharge to the next hospitalization.51
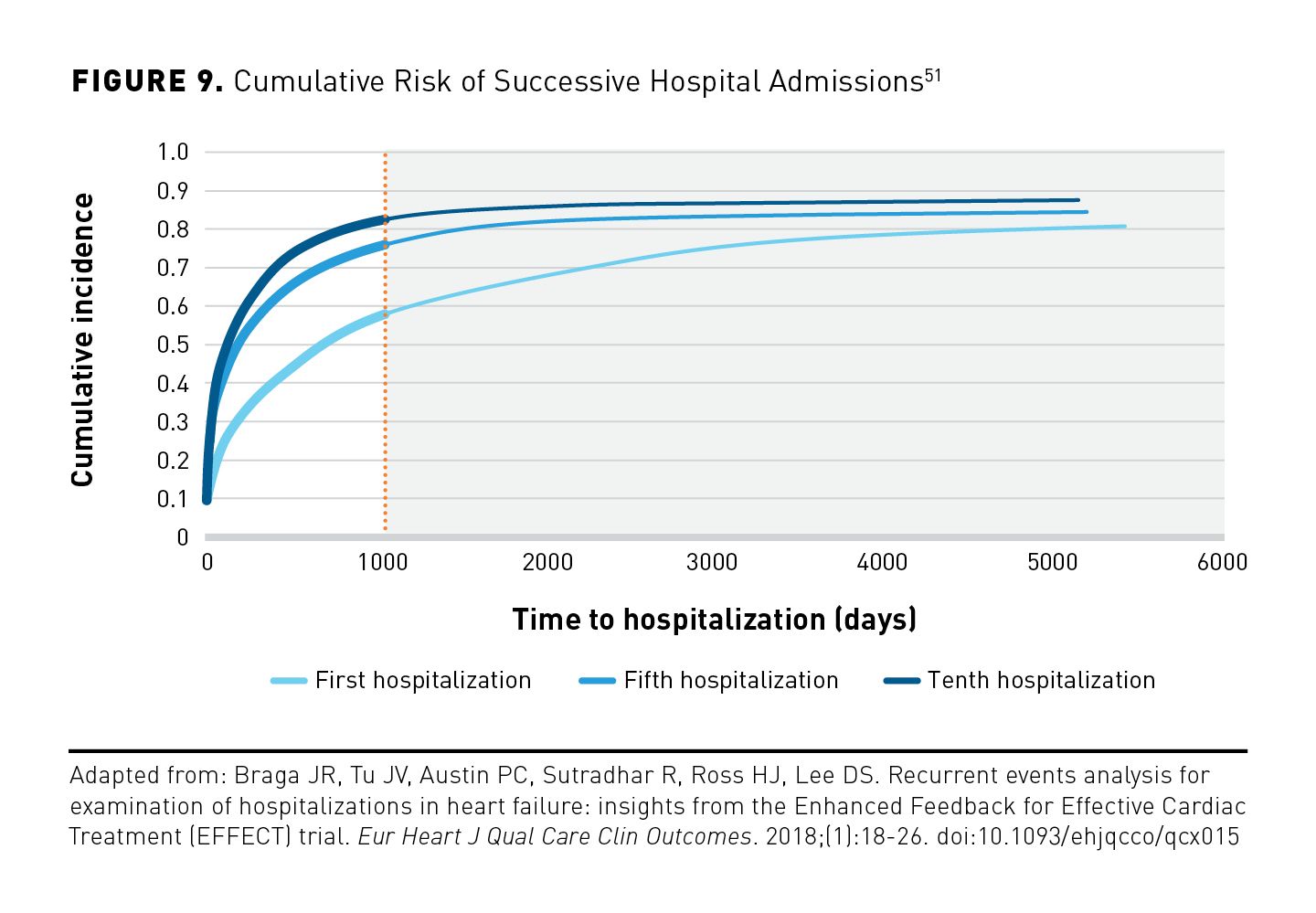
A study of 8948 newly discharged patients with HF that sought to identify predictors of hospital readmission found progressive shortening of the interval between hospitalization episodes. The multivariable analysis consisted of a large cohort of patients with HF and found a sustained association between previous hospitalizations and the rate of subsequent hospitalizations.51
- The interval to subsequent hospitalization progressively decreases over time.52
- Median time to hospitalization decreased from 765 days for the first hospitalization to 226 days for the fifth hospitalization and to 131 days for the tenth hospitalization.52
- Previous hospitalization significantly and independently increased the risk of future hospital admission by 33%.52
Managing Patients With HF After Discharge
Reducing preventable hospitalizations is a national priority; however, this goal has been elusive, and efforts are needed to reduce disease burden and improve outcomes.8 There is a perception that patients treated outside of the hospital setting are clinically more stable; however, ambulatory patients with reduced EF exhibit comparably high relative rates of mortality and hospitalization.19
The trend in recent years is that CMS has cut Medicare payments and that it initiates a different payment structure for unplanned hospital readmissions within 30 days. The trend is continuing; in 2024, Bundled Payments for Care Improvement-Advanced (BPCI-A) will become mandatory. BPCI-A is a value-based payment model from CMS that encourages hospitals to improve communication and care coordination to better engage patients and caregivers in discharge plans and to reduce avoidable readmissions.53 This change makes hospitals and providers financially responsible for the very expensive post–acute care space. This includes all charges related to inpatient rehabilitation, skilled nursing facilities, long-term acute care, and home health services.
Due to the expansion of BPCI-A, 90-day spending will become an increasingly important payment benchmark.54 The first study of its kind examined 90-day spending from 2016 to 2018 Medicare claims for 935,962 patients discharged following HF hospitalization. Despite assumptions that most post-90 day costs would occur in the first 30 days, an even distribution by month seems to occur with two-thirds of costs happening in the second and third months.54 Figure 10 illustrates differences in 90-day hospital readmission rates for HF vs all causes over 7 years.55

Procedure Costs for HF May Be Higher Than Anticipated Due to Complications and Readmissions
Overall, procedures for HF help to save lives, and they may improve QOL for many patients. But these benefits come with a high rate of possible complications.56 LV assist device (LVAD) therapy has become the prevailing surgical treatment (exceeding heart transplantation) for patients experiencing symptoms of advanced HF that are refractory to GDMT.56 LVAD therapy is impactful and is associated with important improvements in survival, functional status, and QOL. For these reasons and because of the limitations of heart transplantation, the number of patients receiving durable LVAD therapy as destination therapy continues to grow.
However, the cost of LVAD therapy is following the same trend. Despite demonstrating survival and QOL improvement in some patients compared with other medical and surgical interventions, LVAD therapy may not be cost-effective. Readmissions can be unpredictable and may be due to complications of device therapy (eg, gastrointestinal bleeding, stroke, device infection, or thrombosis). Readmissions may also occur for reasons unrelated to the LVAD.56 Some data suggest that lower social and economic conditions may be a large driver of readmissions. Social factors (eg, living alone, unmet functional needs, limited education, lack of self-management skills) are most predictive of readmission.56
- In a 2017 study of 220 Medicare patients with LVADs implanted from 2009 to 2010, the mean cost of LVAD implantation was $175,420, and the mean cost of pump replacement was $90,147. Patients had a combined 529 admissions in the year before LVAD implantation and a combined 589 admissions in the year afterward.57
- All-cause readmission after implantation led to $7088 more in costs; hospitalization was 3.5 days longer than before implantation (both, P < .001).57
- Most readmissions resulted from CVD; they were longer and more costly after LVAD therapy than before, particularly admissions for “heart failure and shock with complication/comorbidity” ($12,696 vs $7394, respectively; P < .0001) and for “cardiac arrhythmia and conduction disorders with complication/comorbidity” ($8180 vs $5832; P < .0001).57
The HF Epidemic in the COVID-19 Era
A pandemic within a pandemic, HF was recognized as a global pandemic before the COVID-19 crisis due to its high prevalence worldwide and the likelihood of a dramatic increase in HF as the aging population grows.58 Patients with HF admitted to the hospital with concomitant COVID-19 have a very poor prognosis.59 A retrospective analysis concluded that the risk of mortality in hospitalized HF patients with COVID-19 was higher than the overall risk of inpatient mortality overall (50% vs 23%, respectively).59 It was also found that patients with HF who had COVID-19 were 24% more likely to die in the hospital.59
Out of necessity, the primary focus of hospitals shifted from providing a wide variety of medical care to treating the morbidity and mortality of patients with COVID-19.60 Lower procedure volumes have raised concerns over long-term adverse CV health outcomes resulting from the decreased rate of diagnosis.61 A 2020 study of 909 inpatient and outpatient centers was designed to determine the full magnitude of fewer diagnostic heart disease procedures being performed due to COVID-19 and the impact of this on long-term CVD outcomes. Procedure volumes decreased by 42% from March 2019 to March 2020 and by 64% from March 2019 to April 2020.61
Delays in Care Are Creating a Sense of Urgency
Treatment delays and decreased diagnoses raise serious concerns for long-term adverse CV health, and the long-term impact of comorbid CV risk for those with COVID-19 is still being determined. A 2020 study that investigated immediate consequences of the Danish COVID-19 lockdown for patients with HF found that the usual incidence rates of hospitalization with WHF or of a diagnosis of new-onset HF were reduced by approximately 30% after lockdown.62 These data have raised concerns that patients with HF are currently undertreated, which may negatively impact prognosis in the longer term.63
The rapid adoption of telehealth over the past few years has become important in facilitating continuity of care. Patients at home can be monitored through dedicated applications, telephone calls, or devices. Virtual visits and forward triage allow screening of patients with signs or symptoms of decompensated HF. Patients receiving care in a hospital may benefit from remote communication platforms. After discharge, patients may undergo remote follow-up or telerehabilitation to prevent early readmission.64
Critical Need for Transitional Care Programs for Patients With HF
Transition of care (TOC) in the context of HF management refers to individual interventions and programs with multiple activities designed to improve shifting between therapeutic settings, most often from hospital to home. TOC in HF typically involves home visits by nurses to monitor and manage signs and symptoms and deliver patient education after discharge. Predischarge health education and counseling (eg, discharge planning, health education, discharge counseling, and patient-centered discharge instructions) are important components of TOC intervention.65
TOC in HF requires optimizing communication among stakeholders, identifying patients at high risk, assessing health-related QOL, and ensuring accurate and adequate knowledge for nurses or other clinical leaders. Patient experiences during TOC can be stressful, particularly when posthospitalization care is poorly executed as the result of inadequate coordination of resources or follow-up. Fragmentation of patient care is characterized by ineffective communication among providers and across health care agencies, insufficient patient and caregiver education, poor continuity of care, and limited access—all of which contribute to reduced quality and unfavorable cost outcomes.66
Physiologic, functional, social, cultural, and psychological patient characteristics and unmet needs may also affect HF rehospitalization during TOC. In a study of physical, psychological, social, and existential unmet needs of 132 patients enrolled in cardiac rehabilitation, key points identified were difficulty in motivation to leave home, anxiety when short of breath, general anger and frustration, lack of control of life, depression, unwell feeling, fears of experiencing MI or stroke, forgetting to take medications, lack of understanding about the current situation among family and friends, and obstacles to coping with work around the home.66
As the COVID-19 pandemic demonstrated, acceleration of telehealth strategies can be implemented to ensure continuity of care for patients with HF. Telehealth may increase access and be more convenient for patients with HF who may not be able to receive specialty health care services because of geographic location, physical disability, advanced chronic disease, or difficulty in arranging transportation.67
Drug Classes and Development
Significant scientific breakthroughs in managing HFrEF have been realized in recent decades.67 ARNi (sacubitril/valsartan) has become well established as a first-line agent in HFrEF treatment due to its beneficial impact on cardiac remodeling.68 In addition, vasodilators (eg, vericiguat) stimulate soluble guanylate cyclase and increase cyclic guanosine monophosphate production.67 This may decrease afterload and preload on the myocardium and improve morbidity and mortality in patients with chronic HFrEF.67 SGLT2 inhibitors recently were approved by the FDA to treat patients with type 2 diabetes; their use demonstrated additional benefits, including a positive impact on CV outcomes. SGLT2 inhibitors promote natriuresis, which, combined with diuresis, is associated with decreases in plasma volume, blood pressure, vascular stiffness, preload and afterload, and cardiac wall stress.69
The trajectory of HFrEF has been favorably altered by multiple effective drug therapies. Findings from pivotal clinical trials have led to more individualized therapy for patients with HFrEF.70 Recent developments include use of SGLT2 inhibitors, vasodilators, and transcatheter mitral valve repair, all of which have incrementally improved prognosis beyond that achieved with use of foundational neurohormonal therapies.
When Patients Are Out of Options
For many patients with meaningful comorbidities, limitations in current treatment options present a prevailing challenge. Many patients have reached their individualized limits; however, risks of subsequent hospitalization and death remain significant. In all, HF continues to be a leading cause of hospitalization.22
Unfortunately, multiple factors can have a major impact on the likelihood of patients with HF receiving target doses of GDMT. Lower systolic blood pressure and other unfavorable prognostic factors (eg, more severe NYHA functional class, older age, chronic renal insufficiency, and recent hospitalization for HF) generally occur in most patients not achieving target doses of GDMT.71 In addition to WHF, these factors limit current therapeutic options and success.
Conclusions
Reaching adequate GDMT targets is the foundation of HF treatment. However, many patients do not receive optimal levels of therapy, or they may need more individualized treatment (ie, adjunctive therapy) if their management is not optimized on GDMT. This underscores the need for additional therapeutic options to be used with the current standard of care. A personalized patient approach after initiating GDMT—rather than the more traditional, hierarchical, standardized, one-size-fits-all approach—may lead to more effective therapy for each patient.42
New milestones for the optimal treatment of HF emerge as data are reported, clinical trials continue to resemble the general population more closely, and consideration is given to social determinants, underlying causes of HF, individual patient characteristics, and the specific natural disease course. For example, the emergence of telemonitoring and telehealth during the COVID-19 pandemic led to the adoption of these important modalities to facilitate continuity of care. As HF classification systems become more nuanced, the wait-and-see approach to treatment is being reconsidered, with the hope of shifting the treatment paradigm by using timely and more adaptive interventions. This earlier identification of patients at high risk of poor HF outcomes may be an important step in improving their prognosis.71

Selecting treatment based on patient characteristics (eg, sex, race, genetic predisposition) is also important to decrease rates of mortality and hospitalization.18 Timely interceptive treatments can make significant strides in HF when time is of the essence. As previous “add-on” medications are expanding into frontline use, the need for personalized treatment continues to grow, and new additions to treatment are needed to optimize care and reduce costs.
Improvements in the outpatient care of HF would help discharged patients receive comparable care outside the hospital. Because most patients with WHF have gradual onset of congestive signs and symptoms, there is an extremely important and time-sensitive window of opportunity to halt HF worsening and avoid the need for hospitalization. The coincidental evolution of treatment guidelines and of telemedicine and novel therapeutics on the horizon provides momentum and much needed hope for people with HF.
Acknowledgment: The authors thank Yana Volman and Pierre Forsell of COEUS Consulting Group, LLC, for editorial assistance.
Author Affiliations: VisumRX, LLC (DLC), Portland, OR; Yale New Haven Health System and Yale School of Medicine (NRD), New Haven, CT; Gary Owens Associates (GMO), Ocean View, DE; Humana (former)(CAS), Cincinnati, OH.
Funding Source: Development of this supplement was supported by Cytokinetics, Inc.
Author Disclosures: Dr Clark reports serving as a paid consultant for Cytokinetics and receiving payment for involvement in the preparation of this manuscript. Dr Desai reports serving as a consultant or paid advisory board member for Amgen, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Cytokinetics, Novartis, and Vifor Pharma; he has received grants from Amgen, Boehringer Ingelheim, Cytokinetics, Novartis, and Vifor Pharma; and he has received lecture fees from Amgen, Boehringer Ingelheim, and Cytokinetics. Dr Owens reports serving as a consultant or paid advisory board member for Cytokinetics and ICON; he has received honoraria from the National Association of Managed Care Physicians; and he has received payment for involvement in the preparation of this manuscript. Dr Stemple has no relevant financial relationships with commercial interests to disclose.
Authorship Information: Concept and design (DLC, NRD, GMO, CAS); analysis and interpretation of data (NRD); drafting of the manuscript (NRD, CAS); critical revision of the manuscript for important intellectual content (DLC, NRD, GMO, CAS); administrative, technical, or logistic support (NRD); and supervision (DLC, GMO).
Address Correspondence to: Nihar R. Desai, MD, MPH; Yale New Haven Health System, 330 Cedar St, New Haven, CT 06510. Email: nihar.desai@yale.edu
REFERENCES
1. InformedHealth.org; Institute for Quality and Efficiency in Health Care. Types of heart failure. National Center for Biotechnology Information. January 23, 2018. Accessed June 29, 2022. https://www.ncbi.nlm.nih.gov/books/NBK481485/?report=printable
2. Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9(1):242. doi:10.3390/cells9010242
3. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342-1356. doi:10.1002/ejhf.1858
4. Roger VL. Epidemiology of heart failure. Circ Res. 2013:113(6):646-659. doi:10.1161/CIRCRESAHA.113.300268
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). J Am Coll Cardiol. 2021;77(16):2053-2150. doi:10.1016/j.jacc.2020.11.012
6. Heart failure. Centers for Disease Control and Prevention. Reviewed September 8, 2020. Accessed June 29, 2022. https://www.cdc.gov/heartdisease/heart_failure.htm
7. Cancer Facts and Figures 2022. American Cancer Society; 2022. Accessed June 29, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
8. Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11(12):e004873. doi:10.1161/CIRCHEARTFAILURE.117.004873
9. Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488-504. doi:10.1001/jama.2020.10262
10. Mallick A, Gandhi PU, Gaggin HK, Ibrahim N, Januzzi JL. The importance of worsening heart failure in ambulatory patients: definition, characteristics, and effects of amino-terminal pro-B-type natriuretic peptide guided therapy. JACC Heart Fail. 2016;4(9):749-755. doi:10.1016/j.jchf.2016.03.012
11. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
12. Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019;4(12):1280-1286. doi:10.1001/jamacardio.2019.4187
13. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
14. McDermott KW, Roemer M. Most Frequent Principal Diagnoses for Inpatient Stays in U.S. Hospitals, 2018. HCUP Statistical Brief #277. Agency for Healthcare Research and Quality; 2021. Accessed September 8, 2022. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb277-Top-Reasons-Hospital-Stays-2018.pdf
15. Voigt J, John SA, Taylor A, Krucoff M, Reynolds MR, Gibson MCA. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol. 2014;37(5):312-321. doi:10.1002/clc.22260
16. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. doi:10.116/circ.0000000000001063
17. Solomon SD, Anavekar N, Skali H, et al; Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738-3744. doi:10.1161/CIRCULATIONAHA.105.561423
18. Pandey AP, Omar W, Ayers, C, et al. Sex and race differences in lifetime risk of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Circulation. 2018;137(17):1814-1823. doi:10.1161/CIRCULATIONAHA.117.031622
19. Angaran P, Dorian P, Ha ACT, et al. Association of left ventricular ejection fraction with mortality and hospitalizations. J Am Soc Echocardiogr. 2020;33(7):802-811. doi:10.1016/j.echo.2019.12.016
20. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):P387-P413. doi:10.1016/j.cardfail/2021.01.022
21. Bozkurt B, Khalaf S. Heart failure in women. Methodist Debakey Cardiovasc J. 2017;13(4):216-223. doi:10.14797/mdcj-13-4-216
22. Mentzer G, Hsich EM. Heart failure with reduced ejection fraction in women: epidemiology, outcomes, and treatment. Heart Fail Clin. 2019;15(1):19-27. doi:10.1016/j.hfc.2018.08.003
23. Verhestraeten C, Heggermont WA, Maris M. Clinical inertia in the treatment of heart failure: a major issue to tackle. Heart Fail Rev. 2021;26(6):1359-1370. doi:10.1007/s10741-020-09979-z
24. Papadimitriou L, Moore CK, Butler J, Long RC. The limitations of symptom-based heart failure management. Cardiac Fail Rev. 2019;5(2):74-77. doi:10.15420/cfr.2019.3.2
25. Lala A, Mentz R. Contemplation from our hearts: a call to shift from failure to function. J Card Fail. 2021;27(4):385. doi:10.1016/j.cardfail.2021.03.002
26. Sharma A, Colvin-Adams M, Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleve Clin J Med. 2014;81(5):301-311. doi:10.3949/ccjm.81a.13045
27. Ziaeian B, Fonarow GC, Heidenreich PA. Clinical effectiveness of hydralazine-isosorbide dinitrate in African-American patients with heart failure. JACC Heart Fail. 2017;5(9):632-639. doi:10.1016/j.jchf.2017.04.008
28. Colvin M, Sweitzer NK, Albert NM, et al. Heart failure in non-Caucasians, women, and older adults: a white paper on special populations from the Heart Failure Society of America Guideline Committee. J Cardiac Fail. 2015;21(8):674-687. doi:10.1016/j.cardfail.2015.05.013
29. Maddox TM, Januzzi, JL, Allen LA, et al; Writing Committee. 2021 update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;77(6):772-810. doi:10.1016/j.jacc.2020.11.022
30. Iyngkaran P, Liew D, Neil C, Driscoll A, Marwick TH, Hare DL. Moving from heart failure guidelines to clinical practice: gaps contributing to readmissions in patients with multiple comorbidities and older age. Clin Med Insights Cardiol. 2018;12:1179546818809358. doi:10.1177/1179546818809358
31. Joseph J, Spephy PS, James J, Abraham S, Abdullakutty J. Guideline-directed medical therapy in heart failure patients: impact of focused care provided by a heart failure clinic in comparison to general cardiology out-patient department. Egypt Heart J. 2020;72(1):53. doi:10.1186/s43044-020-00088-8
32. Khan MS, Tahhan AS, Vaduganathan M, et al. Trends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail. 2020;22(6):1032-1042. doi:10.1002/ejhf.1818
33. Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(8):935-944. doi:10.1016/j.jacc.2018.11.049
34. Rosano GMC, Moura B, Metra M, et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23(6):872-881. doi:10.1002/ejhf.2206
35. Brownell NK, Ziaeian B, Fonarow GC. The gap to fill: rationale for rapid initiation and optimal titration of comprehensive disease-modifying medical therapy for heart failure with reduced ejection fraction. Cardiac Fail Rev. 2021;7:e18. doi:10.15420/cfr.2021.18
36. Grewal DG, Partow-Navid R, Garcia D, et al. Role of guideline directed medical therapy doses and optimization in patients hospitalized with decompensated systolic heart failure. Am J Cardiol. 2021;151:64-69. doi:10.1016/j.amjcard.2021.04.017
37. Carnicelli AP, Clare R, Hofmann P, et al. Characteristics and outcomes of patients with heart failure with reduced ejection fraction after a recent worsening heart failure event. J Am Heart Assoc. 2021;10(17):e021276. doi:10.1161/JAHA.120.021276
38. Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy. JAMA Cardiol. 2018;3(3):252-259. doi:10.1001/jamacardio.2017.5250
39. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38(4):406-416. doi:10.1002/phar.2091
40. Parikh KS, Sheng S, Hammill BG, et al. Characteristics of acute heart failure hospitalizations based on presenting severity. Circ Heart Fail. 2019;12(1):e005171. doi:10.1161/CIRCHEARTFAILURE.118.005171
41. Fonarow GC, Stough WG, Abraham WT, et al; OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768-777. doi:10.1016/j.jacc.2007.04.064
42. Cooper LB, DeVore AD, Felker GM. The impact of worsening heart failure in the United States. Heart Fail Clin. 2015;11(4):603-614. doi:10.1016/j.hfc.2015.07.004
43. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardiac defibrillator. J Am Coll Cardiol. 2016;67(9):1062-1069. doi:10.1016/j.jacc.2015.12.046
44. Chang PP, Chambless LE, Shahar E, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113(3):504-510. doi:10.1016/j.amjcard.2013.10.03
45. Mangi MA, Rehman H, Rafique M, Illovsky M. Ambulatory heart failure monitoring: a systemic review. Cureus. 2017;9(4):e1174. doi:10.7759/cureus.1174
46. Borer JS, Bohm M, Ford I, et al; SHIFT Investigators. Efficacy and safety of ivabradine in patients with severe chronic systolic heart failure (from the SHIFT study). Am J Cardiol. 2014;113(3):497-503. doi:10.1016/j.amjcard.2013.10.03
47. McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362(3):228-238.doi:10.1056/NEJMcp090939
48. McMurray JJV, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J. 2012;33(14):1787-1847. doi:10.1093/eurheartj/ehs104
49. Tian J, Yan J, Zhang Q, et al. Analysis of re-hospitalizations for patients with heart failure caused by coronary heart disease: data of first event and recurrent event. Ther Clin Risk Manag. 2019:15:1333-1341. doi:10.2147/TCRM.S218694
50. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
51. Braga JR, Tu JV, Austin PC, Sutradhar R, Ross HJ, Lee DS. Recurrent events analysis for examination of hospitalizations in heart failure: insights from the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) trial. Eur Heart J Qual Care Clin Outcomes. 2018;4(1):18-26. doi:10.1093/ehjqcco/qcx015
52. Hospital readmissions reduction program (HRRP). Centers for Medicare & Medicaid Services. Updated August 25, 2022. Accessed September 8, 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
53. Reinhardt SW, Clark KAA, Xin X, et al. Thirty-day and 90-day episode of care spending following heart failure hospitalization among Medicare beneficiaries. Circ Cardiovasc Qual Outcomes. 2022;15(7):e008069. doi:10.1161/CIRCOUTCOMES.121.008069
54. Khan MS, Sreenivasan J, Lateef N, et al. Trends in 30- and 90-day readmission rates for heart failure. Circ Heart Fail. 2021;14(4):e008335. doi:10.1161/CIRCHEARTFAILURE.121.008335
55. Shih T, Dimick JB. Reducing the cost of left ventricular assist devices: why it matters and can it be done? J Thorac Cardiovasc Surg. 2018;155(6):2466-2468. doi:10.1016/j.jtcvs.2017.12.156
56. Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Heart Fail. 2017;5(2):110-119. doi:10.1016/j.jchf.2016.09.008
57. Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Fail Rev. 2017;3(1):7-11. doi:10.15420/cfr.2016:25:2
58. Chatrath N, Kaza N, Pabari PA, et al. The effect of concomitant COVID-19 infection on outcomes in patients hospitalized with heart failure. ESC Heart Fail. 2020;7(6):4443-4447. doi:10.1002/ehf2.13059
59. Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9(1):65-73. doi:10.1016/j.jchf.2020.11.003
60. Babapoor-Farrokhran S, Alzubi J, Port Z, et al. Impact of COVID-19 on heart failure hospitalizations. SN Compr Clin Med. 2021;3(10):2088-2092. doi:10.1007/s42399-021-01005-z
61. Einstein AJ, Shaw LJ, Hirschfeld C, et al; INCAPS COVID Investigators Group. International impact of COVID-19 on the diagnosis of heart disease. J Am Coll Cardiol. 2021;77(2):173-185. doi:10.1016/j.jacc.2020.10.054
62. Andersson C, Gerds T, Fosbol E, et al. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail. 2020;13(6):e007274. doi:10.1161/CIRCHEARTFAILURE.120.007274
63. Tersalvi G, Winterton D, Cioffi GM, et al. Telemedicine in heart failure during COVID-19: a step into the future. Front Cardiovasc Med. 2020;7:612818. doi:10.3389/fcvm.2020.612818
64. Ba HM, Son YJ, Lee K, Kim BH. Transitional care interventions for patients with heart failure: an integrative review. Int J Environ Res Public Health. 2020;17(8):2925. doi:10.3390/ijerph17082925
65. Albert NM, Barnason S, Deswal A, et al; American Heart Association Complex Cardiovascular Patient and Family Care Committee of the Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8(2):384-409. doi:10.1161/HHF.0000000000000006
66. Schwamm LH, Chumbler N, Brown E, et al; American Heart Association Advocacy Coordinating Committee. Recommendations for the implementation of telehealth in cardiovascular and stroke care: a policy statement from the American Heart Association. Circulation. 2017;135(7):e24-e44. doi:10.1161/CIR.0000000000000475.
67. Pellicori P, Khan MJI, Graham FJ, Cleland JGF. New perspectives and future directions in the treatment of heart failure. Heart Fail Rev. 2020;25(1):147-159. doi:10.1007/s10741-019-09829-7
68. Chim C, Newaz S. SGLT2 inhibitors and heart failure outcomes. US Pharm. 2020;45(2):18-22.
69. Dębska-Kozłowska A, Książczyk M, Lelonek M. Where are we in 2021 with heart failure with reduced ejection fraction? current outlook and expectations from new promising clinical trials. Heart Fail Rev. 2022;27(2):419-430. doi:10.1007/s10741-021-10120-x
70. Greene SG, Butler J, Albert NM. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
71. Clark AL, Cherif M, McDonagh TA, Squire IB. In-hospital worsening heart failure: a clinically relevant endpoint? ESC Heart Fail. 2018;5(1):9-18. doi:10.1002/ehf2.12195

