- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Reducing Economic Burden and Improving Quality of Life in Pulmonary Arterial Hypertension
Abstract
Pulmonary arterial hypertension (PAH) is a rare, progressive disorder associated with a poor prognosis if not treated appropriately. Fortunately, new treatment options have significantly improved survival rates and prognosis. Despite these advances, many patients do not receive the diagnosis until years into their disease or are inappropriately diagnosed. Early referral to specialized treatment centers that allows for early diagnosis and initiation of treatment significantly improves patient outcomes including survival as well as reduction in hospital admissions, which are a main driver of economic burden of disease. It is important that evidence-based guidelines are followed and treatment is individualized based on patient-specific factors. Pharmacologic therapies carry a very high cost for PAH; however, extensive utilization of management strategies may hinder access to medication and may lead to disease progression. Cost containment strategies may help to facilitate care coordination for earlier diagnosis and initiation of treatment, adherence to PAH medications, and patient education to ensure they are using medications appropriately to optimize therapy. Managed care pharmacists can play a crucial role in the multidisciplinary team in terms of medication safety, adherence, patient education, and follow-up to improve patient engagement that leads to improved outcomes.
Am J Manag Care. 2021;27(3):S53-S58. https://doi.org/10.37765/ajmc.2021.88611
Impact of Pulmonary Arterial Hypertension on Survival
Pulmonary arterial hypertension (PAH) is a rare medical condition with a worldwide prevalence estimated at 15 million to 50 million, with 200,000 new cases diagnosed annually in the United States.1 Based on the incidence of PAH, a hypothetical health plan with 5 million patients can expect to have between 60 and 250 patients with PAH, with 12 to 38 new cases annually.2 Symptoms of PAH are similar to other diseases, which may lead to delay of diagnosis.3 Although there have been recent advances in treatment as well as earlier detection and diagnosis of PAH, it is still associated with a poor prognosis. Delayed treatment significantly impacts survival. Prior to the advent of targeted therapies for PAH, median survival from diagnosis to death was estimated at 2.8 years.1
Most epidemiologic data for PAH is from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) and resulting observational studies. This registry consisted of more than 3500 patients in the United States with World Health Organization (WHO) group I PAH. REVEAL data estimated 1-, 3-, 5-, and 7-year survival from diagnosis for patients with PAH to be 85%, 68%, 57%, and 49%, respectively, with median survival of approximately 7 years.4 An analysis aimed to evaluate main predictors of survival found independent variables associated with increased mortality were pulmonary vascular resistance greater than 32 Wood units (hazard ratio [HR], 4.1; 95% CI, 2.0-8.3), PAH associated with portal hypertension (HR, 3.6; 95% CI, 2.4-5.4), modified New York Heart Association/WHO functional class IV (HR, 3.1; 95% CI, 2.2-4.4), men aged older than 60 years (HR, 2.2; 95% CI, 1.6-3.0), and family history of PAH (HR, 2.2; 95% CI, 1.2-4.0).5 New large-scale registries such as the REVEAL, which was completed in 2012, are needed to provide insight of epidemiologic data and survival impact of current treatment therapies for PAH.
Early diagnosis is an important first step to ensure optimal treatment outcomes. It has been reported that patients may come for referral already on one or 2 PAH therapies but have never had a right heart catheterization (RHC). In some cases, the RHC revealed that the patient was misdiagnosed and never had PAH in the first place. Misdiagnosis leads to inappropriate treatment with expensive therapies that may negatively impact patients and incur avoidable economic expenses.2 Unfortunately, misdiagnosis and delayed diagnosis are common because there is no simple test that can be performed to diagnose PAH early in the course of the illness, such as with diabetes and hypertension. On average, it may take 2.3 to 2.8 years for an accurate diagnosis, at which point the disease has likely progressed (Figure 16). Delays in diagnosis result in patients being denied the opportunities for gained survival benefit associated with early and aggressive treatment.7 Additionally, treatment at a later stage of disease is associated with increased hospitalization and length of stay with added costs associated with treating PAH. Therefore, early referral to expert care centers is important for improved outcomes as well as cost-containment strategies associated with PAH.7
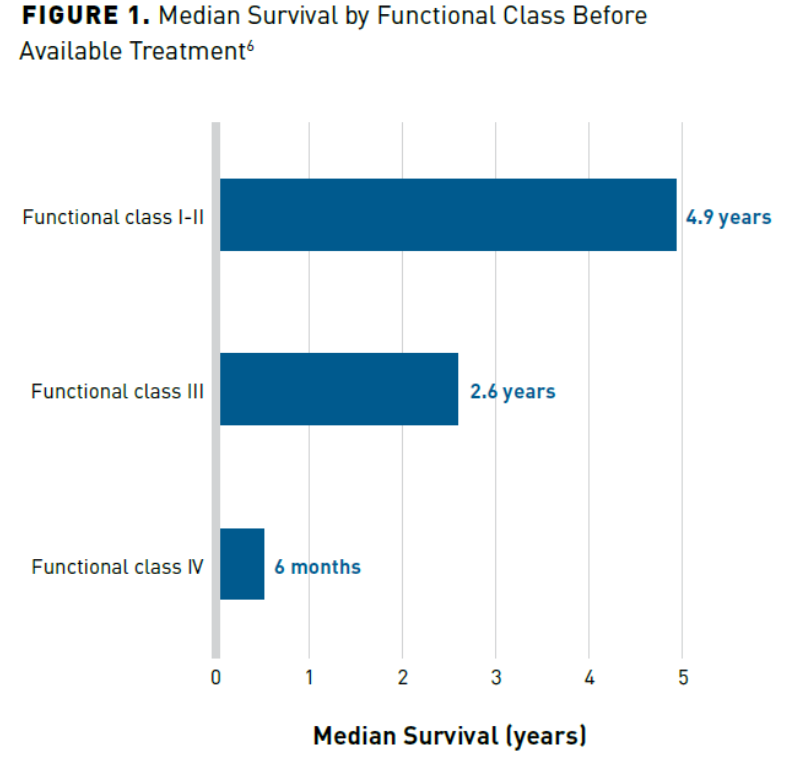
Economic Burden of PAH
The economic impact of PAH can be substantial. Estimated direct per-patient per-month (PPPM) costs for PAH are 4 to 5 times higher than matched control patients with similar age, sex, geographic regions, and employment status.7 A Kaiser Permanente Colorado review found the median total per-patient per-day and 3-year total expenditures for patients with PAH to be $56 and $50,599, respectively.8
A retrospective study was conducted that aimed to determine the correlation of functional decline in patients with PAH with increased healthcare utilization and costs. Data were obtained from Humana Research Database, which contained claims for US commercial and Medicare health plans for approximately 19 million members. Disease severity as indicated by higher functional class was found to be associated with greater likelihood of inpatient episodes and significantly higher PAH-related cost. This suggests patients who progress to advanced functional classes will utilize more services. Therefore, appropriate treatment of the disease to slow progression is a key component in cost mitigation (Figure 26).2 Annual pharmacy costs are estimated to be 17 to 21 times higher than those of the average Medicare part D patient.6
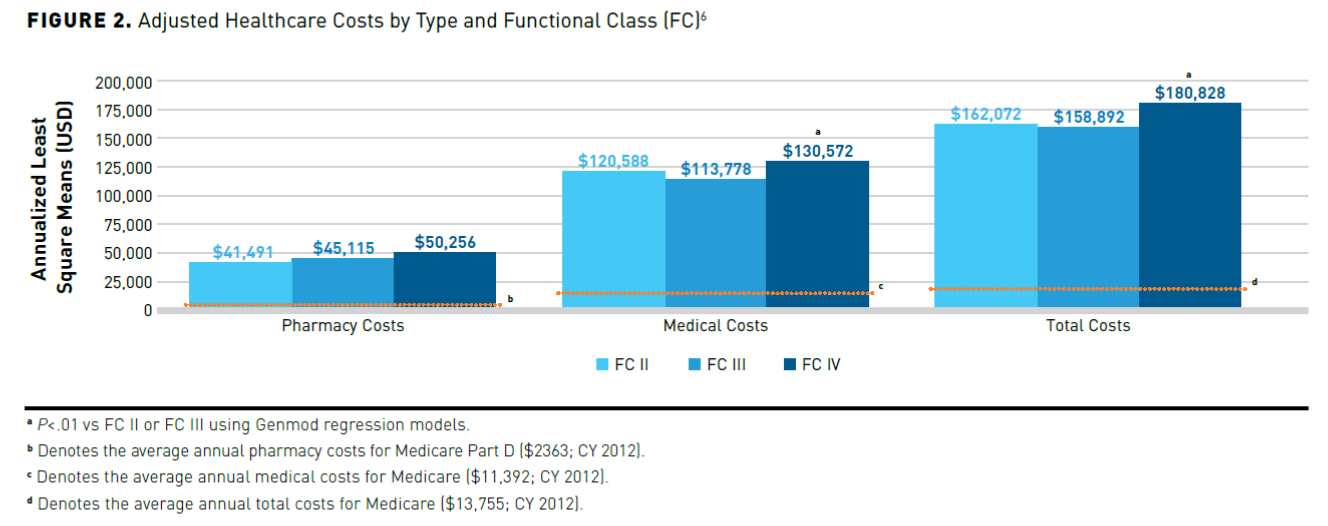
In a claims-based analysis of 504 patients with PAH in a large US managed care plan, researchers evaluated healthcare resource utilization associated with PAH treatment from 2004 to 2010. Patients were followed for 12 months following the index date, defined as the earliest date of a claim of a PAH-indicated medication. Healthcare resource utilization was compared between an annualized baseline period and the 12 months following the index date. Total healthcare costs were significantly lower during the follow-up period compared with the baseline period ($98,243 vs $116,681; P <.001). Pharmacy costs were significantly higher during the follow-up period ($38,514 vs $6440; P <.001). However, medical costs were significantly lower during the follow-up period ($59,729 vs $110,241; P >.001).3
These patients are also at high risk of rehospitalization and 79.3% of hospitalized patients with PAH were found to have a readmission within 1 year of discharge, with 1 in 5 patients having a readmission within 30 days of discharge.9 The average cost of patients with at least 1 hospitalization totaled $46,118 (SD, $135,137), while readmission totaled $35,188 (SD, $152,006).9
There is a growing body of evidence in support of early initiation of combination therapy.8 A systematic review examined drug cost, hospitalization burden, healthcare economics, and the effect of early intervention on clinical outcomes. Evaluating studies from 2005 to 2017, findings indicated that early therapy is effective. Results also showed benefit of combination therapy with a decrease in hospitalization, particularly in patients with FC II, but noted drug costs increased with combination therapy. A review of pharmacoeconomic studies indicated that an increase in pharmacy costs were at least partially offset by decreased healthcare resource utilization, particularly inpatient visits.8 There is evidence that PAH treatment decreases hospitalization frequency, which in turn may lead to reduced healthcare utilization costs associated with PAH. As shown in the AMBITION trial, combination therapy with ambrisentan plus tadalafil compared with pooled monotherapy decreased hospitalizations due to PAH (4% with combined therapy vs 12% with monotherapy). Additionally, results of the SERAPHIN trial indicate that macitentan decreased hospitalizations due to PAH by 50% (HR, 0.50; 97.5% CI, 0.34-0.75). Furthermore, the risk of all-cause hospitalizations decreased by 19% and 32% in the 3-mg and 10-mg daily dosing arms compared with placebo. Risk of PAH-related hospitalizations was reduced by 43% (P <.001) and 52% (P <.001) in the 2 treatment arms.8
Encouraging Appropriate Medication Use and Application of Evidence-Based Guidelines
Patients with PAH are typically fragile and have multiple comorbidities, necessitating an individualized management approach based on their risk and disease progression.9 Current evidence suggests there are a variety of treatment options for patients with PAH including general guidance (eg, physical activity, oral anticoagulants, psychosocial support), nonspecific pharmacologic interventions (eg, calcium channel blockers), as well as targeted pharmacologic interventions depending on patient-specific factors.9
There is growing evidence to support early aggressive treatment with multiple therapies for treatment-naive patients. One study analyzed the effect of initial triple combination therapy (including intravenous or subcutaneous prostacyclin and 2 oral drugs) on long-term survival in patients newly diagnosed with PAH. Patients initiated with triple combination had more severe PAH compared with those on dual combination or monotherapy (P <.001). In patients initiated with triple combination therapy, the actual versus predicted overall survival rates at 1, 2, and 3 years were 94%, 92%, and 90%, versus 76%, 62%, and 51%, respectively. The use of triple combination therapy was shown to reduce the risk of death compared with mono- or dual-combination therapy (HR, 0.35; 95% CI, 0.17-0.71; P = .003).10
A 2016 meta-analysis included prospective, randomized, controlled trials of at least 12 weeks duration between 1990 and 2015 that compared combinations of approved PAH treatments with monotherapy having primary end points of clinical worsening or one or more secondary outcomes (all-cause mortality, PAH-related mortality, PAH-related admission to hospital, lung transplantation, treatment escalation, symptomatic progression, changes in WHO functional class, treatment discontinuation, and treatment duration). The meta-analysis included 17 studies (4095 patients) and found that combination therapy significantly reduced the risk of clinical worsening when compared with monotherapy (risk ratio, 0.65 [95% CI, 0.58-0.72]; P <.00001). Results were consistent among subgroups. Additionally, functional status was also improved with combination therapy. Data from this study strongly favor the use of combination therapy and have shaped treatment guidelines over time.
The REVEAL Registry has indicated that 46% of patients with PAH are on dual therapy, and 9% of patients are on triple therapy.9 While combination therapy does elicit favorable results in many patients, the guidelines do recognize that not all patients with PAH may benefit from combination therapy and monotherapy is an appropriate option in some patients. This demonstrates a need for new and innovative treatment options for PAH, and access to current therapies in the interim.9 Pharmacy wholesale acquisition costs range from $25,000 to $250,000 for “average dosing” of PAH medications.9 These high prices have made payers interested in utilization management strategies including prior authorization, tiered use, and formulary restrictions. However, cost offsets could be achieved by earlier diagnosis of the disease and appropriate targeted treatment.
An economic model examined the potential impact of selexipag. The model assumed 1 million health plan members and a 2-year horizon calculated annual costs with 2 separate scenarios: 1 with selexipag and 1 without. The model predicted 22 patients with PAH in year 1 and 22 patients with PAH in year 2 would be treated with a prostacyclin pathway. They predicted the 2-year cost would be offset by a 15% reduction in costs with the addition of selexipag, which would result in a cost-savings of $0.04 per member per month (PMPM).8
It is critical that healthcare providers continue to tailor treatment regimens to the individual patient. Guidelines recommend the optimal approach is individualized based on severity of illness, route of administration of therapy, adverse effect profile, comorbid illnesses, treatment goals, and clinician experience and preference. Due to this individualized approach, patients should be able to access clinically appropriate and approved treatments for PAH to allow for optimized outcomes. It is important to remember that PAH is a progressive disease and delays in initiation of optimal treatment regimens may be associated with deterioration in functional class, which has been a predictor of mortality.9 If patients must move through a tier-by-tier restrictive utilization process, many patients may not recover the time lost or may experience complications due to comorbidities. Formulary exclusions may also impact optimal treatment when there are drug shortages. The appeal process for noncoverage or nonformulary items comes at a high cost due to wasted time and administrative burden. The 2020 Council for Affordable Quality Healthcare index estimates that overall spending on prior authorizations is $767 million in the United States, with 86% of the spending incurred by providers.11 As many as 79% of prescribers report that they must resubmit appeals for repeated prescriptions for chronic conditions such as PAH. Rather than intensive utilization management strategies on PAH medications, it may be more appropriate to ensure these patients are being closely followed by care management and care coordination services.6 Due to the progressive nature of the disease and the supporting literature that early, combination therapy has proven to demonstrate a benefit in terms of mortality, hospitalizations, and disease progression, payers should maintain flexibility to help facilitate an individualized treatment approach. Utilization management strategies that are too restrictive and add more time to receive therapies may negatively impact disease outcomes for progressive orphan diseases such as PAH.9
Educating Healthcare Providers and Patients With PAH
A patient-centered approach that takes into account the impact of illness on day-to-day activities and quality of life and aims to improve these is an important strategy for improved outcomes in PAH. Guidelines recommend early referral to expert centers to accurately diagnose and initiate treatment. According to the 2015 European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines, the role of providers within an expert center is to assess and investigate all causes of PAH, manage PAH-specific therapies, and work collaboratively with other healthcare providers, including primary care, in order to optimize patient outcomes.7 Despite educational initiatives, referrals to care centers are often delayed and continue to be problematic. Patients are often referred with incomplete diagnostic testing, misdiagnosis, inappropriate treatments, or are not referred until their disease has progressed significantly.7 PAH is often diagnosed after symptoms have begun and patients may present with only vague symptoms including shortness of breath, fatigue, and activity limitation. They may also be inappropriately diagnosed with asthma or chronic obstructive pulmonary disease.6
The lack of referrals for patients with PAH to care centers has led to the development of the Pulmonary Hypertension Care Center committee, which aspires to improve the overall quality of care and outcomes of patients with PAH. This committee oversees an accreditation program that promotes standards and adherence to guidelines as well as fosters collaboration among experts. These initiatives may potentially improve patient referrals to expert care and contribute to improved outcomes as well as cost containment. While early referral to expert care centers should remain a primary strategy, there is still a growing need for education. Healthcare providers including local primary care and specialty physicians should work together with care centers. Nonadherence to treatment guidelines leads to patients being undertreated, especially those patients with more progressed disease, who are often treated with inappropriate medication options.7
Managed care professionals can successfully engage patients through education and communication of high-quality information. There is growing evidence that patient engagement generally improves outcomes. Patients with PAH who are better informed find it easier to manage their disease in key areas such as health-related quality of life, health status, anxiety, depression, and self-management. A wealth of information is available on the internet, but there is potential for significant misinformation as well. It is important that disease information is communicated accurately about the patient’s specific circumstances and disseminated at the appropriate time.12 Additionally, the growing use of early aggressive combination therapy creates the need for greater monitoring and management of adverse effects (AEs).7 Pharmacists are vital educators for patients on managing their disease, monitoring for AEs, and ensuring patients understand their medications. Additionally, with combination therapy now being routinely used, it may complicate a patient’s treatment regimen and impact medication adherence.13 Another challenge is that PAH may impair cognitive function, affecting verbal learning that may limit a patient’s engagement in the management of their disease. A further challenge is that patients may not want to be engaged in the management of their disease as a coping mechanism to avoid dealing with the harsh realities of their diagnosis. It is important for healthcare professionals to communicate to patients the benefits that engagement can have on outcomes and to encourage participation.12
The ESC/ERS guidelines recommend increasing patient engagement through collaboration with other healthcare professionals, including psychologists, psychiatrists, social workers, and patient associations. For patients in rural areas unable to receive the type of care usually provided by large-scale healthcare institutions, it is important for local family physicians to collaborate with other healthcare professionals in the local community. This collaborative approach can optimize patient engagement from a multitude of different standpoints. Additionally, receiving information from a diverse team of healthcare professionals may be beneficial because each individual may convey information differently.12
Another vital role for managed care professionals in a healthcare team is education on medication administration and safety. Specifically, parenteral prostanoids have a risk of medication errors and AEs. Epoprostenol is included on the Institute for Safe Medication Practice’s list of high-alert medications. One survey has shown that of reported errors associated with this therapy, two-thirds were serious or potentially serious. Fatalities were also reported. Errors can include accidental bolus dosing (flushing the dedicated line), incorrect dosing (miscalculation, compounding, ordering errors), and pump-related errors. Pharmacists are needed to develop policies that reduce the risk of error and educate providers to ensure safe and appropriate medication use. This may include double checking calculations, confirming dosing with the patient’s specialty pharmacy, minimizing storage of backup cassettes or bad preparation, and the use of different colored cassettes for different agents if available. Pharmacists can also help optimize therapy by ensuring appropriate dosing and titration of prostanoids, as dose tolerability is limited by patient-reported AEs. Pharmacists can also assist with drug−drug interactions, transitions between PAH therapies, and management of medications for other AEs.1
Assisting With Barriers Related to Medication Acquisition and Specialty Pharmacy
Specialty pharmacies can play a crucial role in the coordination of care for patients with PAH and mitigate medication-related barriers. Specialty pharmacists can improve drug adherence and outcomes for patients that optimizes their overall quality of life. In this setting, pharmacists can assist with insurance approval, patient counseling, overcoming financial constraints, and managing AEs.14 The specialty pharmacy model has resulted in decreased provider and clinic burden, decreased time for medication approval and initiation, patient and provider satisfaction, and patient cost savings by facilitating and improving access to manufacturer discount programs. For these reasons, there is a rapid growth of specialty pharmacies for conditions that need to be more closely followed.15
Managed care professionals in the specialty care setting can also play an important role in impacting medication adherence. These therapies are very costly, so it is even more crucial to ensure that patients are taking the medications appropriately. Additionally, closely following these patients may play an important role in preventing rehospitalizations, for which patients with PAH are at high risk. One retrospective study included adult patients with PAH who were prescribed phosphodiesterase-5 inhibitor therapy by the center’s outpatient clinic and who received medication management through the center’s specialty pharmacy. Adherence was defined as proportion of days covered (PDC) greater than or equal to 80%. Of the 131 patients included in the study, 94% achieved optimal medication adherence greater than or equal to 80% of PDC. Of the total patients, 47% experienced an AE and 27% had at least 1 hospitalization. Patients with a PDC less than 80% were more likely to experience an AE when compared with patients with PDC greater than or equal to 80% (P = .002).14
Another aspect of PAH medication is regulatory compliance. Risk Evaluation and Mitigation Strategy programs exist for endothelin receptor antagonists as well as riociguat. These medications are only available through certified outpatient specialty pharmacies to manage their monitoring and compliance requirements.16 Specialty pharmacy’s care coordination allows access to medications with AE concerns (eg, embryo-fetal toxicity, hepatotoxicity) while still maximizing patient safety.
Conclusions
New therapies for PAH have significantly changed the landscape of available treatment options. Studies and guidelines now support use of combination therapies in appropriate patient populations. Although combination therapy is associated with significant pharmacy costs, there is evidence that such treatment can reduce hospitalizations, which is a main contributor of healthcare costs associated with PAH. Managed care pharmacists can play a key role in patient and provider education of therapies including dosing, medication counseling, and medication safety. Specialty pharmacies can also have a significant role in improving the care of patients with PAH by facilitating access to medication, improving medication adherence, and ensuring that appropriate safety and regulatory requirements are being followed. Generic medications may also impact treatments and managed care pharmacists can be a key resource in answering the many questions patients may have on generic availability and their impact.
Author affiliation: Douglas S. Burgoyne, PharmD, FAMCP, is adjunct associate professor, Department of Pharmacotherapy, University of Utah College of Pharmacy, Salt Lake City, UT.
Funding source: This activity is supported by an educational grant from United Therapeutics Corporation.
Author disclosure: Dr Burgoyne has no financial relationships with commercial interests to disclose.
Authorship information: Acquisition, analysis, and interpretation of data; concept and design; critical revision of the manuscript for important intellectual content.
Address correspondence to: burgoyned@gmail.com
Medical writing and editorial support provided by: Jenna Wood, PharmD
References
1. Macaulay TE, Covell MB, Pogue KT. An update on the management of pulmonary arterial hypertension and the pharmacist’s role. J Pharm Pract. 2016;29(1):67-76. doi: 10.1177/0897190015615902
2. Studer S, Hull M, Pruett J, Koep E, Tsang Y, Drake W 3rd. Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Pulm Circ. 2019;9(1):2045894018816294. doi: 10.1177/2045894018816294
3. Sikirica M, Iorga SR, Bancroft T, Potash J. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;14:676. doi: 10.1186/s12913-014-0676-0
4. McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8-18. doi: 10.1183/09059180.00008211
5. Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164-172. doi: 10.1161/CIRCULATIONAHA.109.898122
6. Studer SM, Kingman M, Calo L, et al. Considerations for optimal management of patients with pulmonary arterial hypertension: a multi-stakeholder roundtable discussion. Am J Manag Care. 2017;23(suppl 6):S95-S104.
7. Hill NS, Cawley MJ, Heggen-Peay CL. New therapeutic paradigms and guidelines in the management of pulmonary arterial hypertension. J Manag Care Spec Pharm. 2016;22(3-a):S1-S16.
8. Burger CD, Ghandour M, Padmanabhan Menon D, Helmi H, Benza RL. Early intervention in the management of pulmonary arterial hypertension: clinical and economic outcomes. Clinicoecon Outcomes Res. 2017;9:731-739. doi: 10.2147/CEOR.S119117
9. Highland KB, Hughes KE, Williams KJ, Kyei-Baffour B, Ferguson S. Ensuring appropriate access to pulmonary arterial hypertension therapy. Am J Manag Care. 2019;25:S119-S127. Accessed February 22, 2021. cdn.sanity.io/files/0vv8moc6/ajmc/9f016dbbead2ba8d9841dab2b4d9bc8e80d3e2dc.pdf
10. Boucly A, Savale L, Weatherald JC, et al. Impact of initial triple combination therapy on long-term survival in pulmonary arterial hypertension (PAH). Am J Respir Crit Care Med. 2020;201:A5585.
11. CAQH 2020 index. Closing the gap: the industry continues to improve but opportunities for automation remain. 2021. Accessed February 21, 2021. caqh.org/sites/default/files/explorations/index/2020-caqh-index.pdf
12. Graarup J, Ferrari P, Howard LS. Patient engagement and self-management in pulmonary arterial hypertension. Eur Respir Rev. 2016;25(142):399-407. doi: 10.1183/16000617.0078-2016
13. Grady D, Weiss M, Hernandez-Sanchez J, Pepke-Zaba J. Medication and patient factors associated with adherence to pulmonary hypertension targeted therapies. Pulm Circ. 2018;8(1):2045893217743616. doi: 10.1177/2045893217743616
14. Shah NB, Mitchell RE, Proctor ST, et al. High rates of medication adherence in patients with pulmonary arterial hypertension: an integrated specialty pharmacy approach. Plos One. 2019;14(6): e0217798. Accessed February 22, 2021. journals.plos.org/plosone/article?id=10.1371/journal.pone.0217798
15. Bagwell A, Kelley T, Carver A, Lee JB, Newman B. Advancing patient care through specialty pharmacy services in an academic health system. J Manag Care Spec Pharm. 2017;23(8):815-820. doi: 10.18553/jmcp.2017.23.8.815
16. Wong, BA. CEO update: Introduction of generic drugs changing therapy landscape for many. June 5, 2019. Accessed January 2, 2021. phassociation.org/ceo-update-introduction-of-generic-drugs-changing-therapy-landscape-for-many
