- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Recent Developments in the Treatment of Advanced or Recurrent Cervical Cancer
Cervical cancer is one of the most common gynecologic tumors diagnosed worldwide, and most early-stage cases can be successfully treated with surgery and, often, concurrent chemoradiotherapy.1-4 In the United States, however, there are racial and socioeconomic disparities in cervical cancer screening, incidence, and mortality.5,6 Results from an analysis of US cervical cancer incidence data from 2010 to 2014 (59,432 cases) found that women living in rural areas had higher rates of localized, regional, and distant disease than did women residing in urban areas. Furthermore, incidences of regional and distant disease were higher among Hispanic and Black women than among White women.5 These disparities may be exacerbated in the near future as a result of decreased access to screening and preventive care that has occurred during the COVID-19 pandemic.6
For patients with recurrent, persistent, or metastatic cervical cancer, there are limited therapeutic options, and the 5-year overall survival (OS) is approximately 15.0%. In 2014, bevacizumab was added to chemotherapy regimens as a first-line treatment for this patient population, which resulted in nearly a 4-month increase in OS. However, there is still a need for more effective options for the second-line setting and beyond. Targeted approaches, including the use of immune checkpoint inhibitors, are currently being investigated for the treatment of these patients. In particular, use of PD-1 and PD-L1 inhibitors has achieved favorable efficacy and may be a promising approach for cervical cancer treatment.4,7
PD-1/PD-L1 Inhibitors in Cervical Cancer
Various immunomodulatory therapies are under investigation in clinical trials, but only CTLA4 inhibitors and PD-1/PD-L1 inhibitors have been approved by the FDA for cervical cancer. CTLA4 integrates with molecules CD80 and CD86, which are present on antigen-presenting cells. PD-L1 also expresses on tumor cells, tumor-associated fibroblasts, and other cell types. Therefore, CTLA4 inhibitors regulate T cells within secondary lymphoid organs, whereas PD-1/PD-L1 inhibitors regulate T cells within peripheral tissues and the tumor microenvironment. Because targeting the PD-1/PD-L1 signaling pathway is more specific to the tumor itself, use of PD-1/PD-L1 inhibitors rather than CTLA4 inhibitors may result in less damage to healthy tissues.7
The role of the PD-1/PD-L1 immune-checkpoint pathway in cancer immune evasion has been extensively studied. Various solid tumors, including cervical cancer, use this as a mechanism to inhibit the immune system.7,8 PD-1–positive tumor infiltrating lymphocytes (TILs) were identified in 47.0% of patients with cervical cancer in one study, whereas another found that 60.8% of stromal cells from patients with cervical cancer were PD-1 positive.7,9,10 Similarly, between 33.0% and 96.0% of cervical cancer cells have higher PD-L1 expression than found at baseline.7,11 This increase in PD-L1 expression by cervical cancer tumor cells has been correlated with human papillomavirus (HPV) infection.7,12,13 Therefore, the PD-1/PD-L1 pathway is a promising immunotherapeutic target for cervical cancer. In addition to pembrolizumab, which is already FDA approved, several other PD-1/PD-L1 targeted therapies have shown potential for use in patients with recurrent or advanced cervical cancer.7,14,15
Atezolizumab
Atezolizumab is a monoclonal antibody that targets PD-L1, blocking its interaction with PD-1 and the costimulatory molecule CD80.14 It is indicated for the treatment of urothelial carcinoma, triple-negative breast cancer, non–small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma (HCC), and melanoma.16
The therapeutic value of atezolizumab plus bevacizumab for the treatment of advanced cervical cancer was explored in a phase 2, multicenter, single-arm clinical trial sponsored by the National Cancer Institute (NCT02921269). In the analysis, 11 patients were treated with a combination of 15 mg/kg bevacizumab and 1200 mg atezolizumab given intravenously (IV) every 3 weeks. The primary end point, assessed by a Simon 2-stage phase 2 design, was objective response rate (ORR), with a desirable response rate of 0.40. At least 2 of 10 patients needed to demonstrate a response in the first stage for the study to advance to stage 2. At the time of the efficacy analysis, all patients in the evaluable population (n = 10) had died; the confirmed ORR was 0.0%. Thus, the combination of bevacizumab and atezolizumab did not meet the predefined efficacy end point. Notably, 2 patients achieved an unconfirmed partial response (PR) at the first or second scans but progressed afterward. The disease control rate (DCR) was 60.0% (complete response [CR], 0.0%; PR, 0.0%; stable disease, 60.0%), with a median progression-free survival (PFS) of 2.9 months and OS of 8.9 months.14
The safety analyses included all 11 patients, and the overall rate of grade 3 or 4 treatment-related adverse events (TRAEs) was 36.4%. No treatment-related deaths occurred. Two patients discontinued treatment after developing potentially treatment-related grade 3 neurologic events; 1 patient had encephalopathy and meningitis, and the other had arachnoiditis. Overall, the most commonly occurring AEs were hypertension (18.0%) and gastrointestinal (GI) fistula (18.0%). The most common TRAEs included fatigue (36.0%), increased aspartate aminotransferase level (27.0%), nausea (27.0%), fever (27.0%), increased alanine aminotransferase level (18.0%), diarrhea (18.0%), and dyspnea (18.0%).14
Cemiplimab
Cemiplimab, a human PD-1–blocking monoclonal antibody, is the first and only agent indicated to date for the treatment of patients with locally advanced or metastatic basal cell carcinoma who cannot be treated with a Hedgehog pathway inhibitor because of previous treatment or intolerance.17 It is also indicated as a first-line treatment option for patients diagnosed with NSCLC with high PD-L1 expression and no EGFR, ALK, or ROS1 abnormalities, as well as for cutaneous squamous cell carcinoma (SCC). As a PD-1 blocking agent, cemiplimab prevents binding of PD-L1 and PD-L2 and allows T cells to mount an immune response against the tumor.18,19
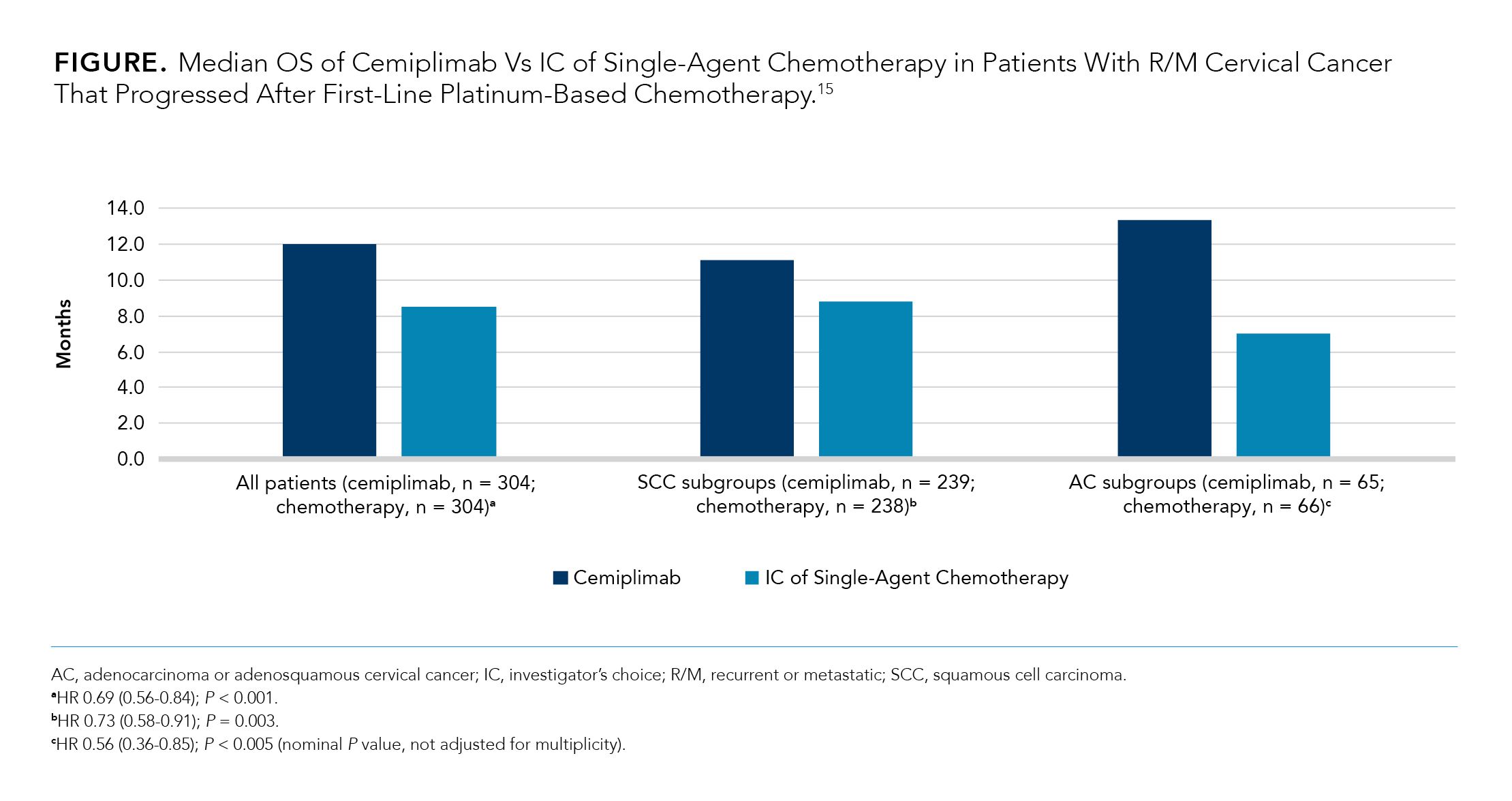
The open-label, randomized, phase 3 EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 (NCT03257267) clinical trial compared cemiplimab with investigator’s choice (IC) single-agent chemotherapy for recurrent or metastatic (R/M) platinum-refractory cervical cancer. The trial ended early based on positive results demonstrating a benefit in OS, which was the primary end point.15,20,21
The study enrolled 608 patients, regardless of PD-L1 expression. Of these, 477 patients had cervical SCC, and 131 patients had adenocarcinoma (ADC). The median age of patients was 51 years, and they all had ECOG performance status (PS) of 0 or 1. Patients received either 350 mg IV of cemiplimab every 3 weeks or IC of chemotherapy (pemetrexed, vinorelbine, gemcitabine, irinotecan, or topotecan) for up to 96 weeks.15
As shown in the Figure,15 the results of this study demonstrated that patients treated with cemiplimab (n = 304) experienced significantly longer median OS than patients treated with IC of single-agent chemotherapy (n = 304), regardless of PD-L1 status (12.0 months vs 8.5 months; HR 0.69, 0.56-0.84; P < 0.001). Among the patient subgroups, patients with ADC or adenosquamous cervical cancer experienced the longest median OS (13.3 months) when treated with cemiplimab (n = 65) but the shortest median OS (7.0 months) when treated with IC of single-agent chemotherapy (n = 66).15
The most common AEs of any grade experienced by patients treated with cemiplimab vs IC of single-agent chemotherapy were anemia (25.0% vs 45.0%, respectively), nausea (18.0% vs 33.0%), and vomiting (16.0% vs 23.0%). The rate of AE-related discontinuations was 8.0% for cemiplimab and 5.0% for IC of chemotherapy.15
Based on these results, a regulatory submission for a cervical cancer indication for cemiplimab is expected in 2021.20
Nivolumab
Nivolumab is also a human PD-1–blocking monoclonal antibody; to date, it is indicated for the treatment of 13 types of cancer, including melanoma, NSCLC, SCC of the head and neck (SCCHN), and HCC.22,23
The efficacy and safety of nivolumab for the treatment of patients with R/M cervical, vaginal, or vulvar cancers were assessed in the phase 1/2 CheckMate 358 clinical trial (NCT02488759). The study enrolled 24 adult patients with an ECOG PS of 0 or 1 and R/M disease who had received up to 2 prior systemic therapies. In the cervical SCC cohort (n = 19), the median age was 51.0 years; most patients had stage IV disease (84.2%), metastases in lymph nodes and lungs (40.0%), HPV-positive tumors (83.3%), and PD-L1 expression of at least 1% (62.5%).24
Patients received 240 mg of nivolumab IV every 2 weeks for up to 2 years; the median duration of treatment was 5.6 months. The median follow-up for patients in the cervical SCC cohort was 19.2 months. The primary end point of investigator-assessed ORR in the cervical SCC cohort was 26.3% (DCR, 68.4%). As for secondary end points, the median duration of response (DOR) was not reached; the median OS was 21.9 months (OS rate at 24 months, 49.8%). The median PFS was 5.1 months (PFS rate at 12 months, 26.3%). Furthermore, PD-L1 status or prior systemic therapy did not considerably affect OS.24
No treatment-related deaths were reported, and the rate of grade 3 or 4 TRAEs (including diarrhea, hepatocellular injury, and pneumonitis) in the cervical SCC cohort was 15.8%. The most common TRAEs were GI events (21.1%) and skin reactions (21.1%).24
Durvalumab
Durvalumab is a human monoclonal antibody that binds PD-L1 and CD80 and blocks PD-L1 activity.25,26 It is indicated to treat patients with unresectable, stage III NSCLC and as a first-line treatment for extensive-stage SCLC.25
The ongoing phase 3 CALLA clinical trial (NCT03830866) is evaluating the efficacy and safety of durvalumab used with and following concurrent chemoradiotherapy as compared with concurrent chemoradiotherapy alone in patients with stage IB2 (cancer is still in the cervix and is 2 cm to 4 cm in size) through stage IVA cervical cancer (cancer has spread to nearby organs, such as the bladder or rectum).25-28
In addition to chemoradiotherapy (cisplatin or carboplatin plus external-beam radiotherapy followed by image-guided brachytherapy once weekly for 5 weeks), patients will receive either 1500 mg of durvalumab or placebo every 4 weeks for 24 cycles. The study will enroll fit, adult patients with histologically confirmed and previously untreated cervical ADC, cervical SCC, or adenosquamous carcinoma that is stage IB2 to IIB (node-positive) and IIIA to IVA (any node stage) with no evidence of metastases.28
The primary end points for this study include PFS based on investigator-assessed progression, histopathological progression, or death. Secondary end points include 3-year PFS, PFS in PD-L1–positive patients, ORR, CR, distant disease progression, secondary malignancy, QOL, and safety.26
The estimated primary completion date is October 27, 2022.28
Pembrolizumab
In 2014, pembrolizumab was the first human PD-1–blocking antibody to be approved by the FDA and, as of November 2021, is indicated to treat 18 types of cancer, including NSCLC, SCCHN, and HCC.29
Pembrolizumab monotherapy is indicated for the treatment of patients with R/M cervical cancer with PD-L1 expression (combined positive score [CPS] ≥ 1).29,30
The KEYNOTE-826 clinical trial (NCT03635567) assessed the safety and efficacy of adding pembrolizumab to chemotherapy with or without bevacizumab as a first-line treatment for advanced cervical cancer. The study enrolled fit, adult patients with previously untreated, measurable, and persistent, recurrent, or metastatic ADC, adenosquamous carcinoma, or SCC of the cervix that was not amenable to curative treatment.31
A total of 617 patients received either pembrolizumab or placebo in addition to chemotherapy with or without bevacizumab. Of these patients, 548 had a PD-L1 CPS of 1 or more, and 317 had a PD-L1 CPS of 10 or more. The median PFS, a primary end point, was significantly longer in the pembrolizumab group in patients with a CPS of at least 1 (10.4 vs 8.2 months with placebo, respectively; HR for disease progression or death, 0.62; 95% CI, 0.50-0.77; P < .001), in patients with a CPS of at least 10 (10.4 vs 8.1 months; HR, 0.58; 95% CI, 0.44-0.77; P < .001), and in the intent-to-treat (ITT) population (10.4 vs 8.2 months; HR, 0.65; 95% CI, 0.53-0.79; P < .001). The median OS was not reached in the PD-L1–selected populations, was 24.4 months in the ITT population, and was 16.3 to 16.5 months in the placebo group.31
Various AEs were reported in both the pembrolizumab and the placebo groups, with the most common of any grade being anemia, alopecia, neutropenia, decreased neutrophil count, and hypertension. Common AEs that occurred in at least 10% of patients for which there was a greater risk in the pembrolizumab group than the placebo group were hypothyroidism (18.2% vs 9.1%, respectively) and decreased white blood cell count (12.1% vs 7.1%). Patients in the pembrolizumab group experienced a higher rate of potentially immune-mediated AEs than did patients in the placebo group (33.9% vs 15.2%). One patient in the pembrolizumab group died from encephalitis.31
Experimental Therapies in Cervical Cancer
In addition to research into the growing plethora of immunotherapeutic agents described above, there is also the development of other promising innovative approaches to treat advanced and metastatic cervical cancer. Some of these approaches, such as adoptive T-cell therapy, antibody-drug conjugates, and DNA damage response inhibitors, have been tested in clinical trials for the treatment of advanced cervical cancer.4,32-37
Adoptive T-cell Therapy
Adoptive T-cell therapies are an approach whereby lymphocytes are isolated from the patient or a donor, manipulated and expanded ex vivo, and reinfused into the patient in large quantities to target viral or tumor antigens.4,32 Various studies have investigated the potential of adoptive T-cell therapy for advanced cervical cancer using both tumor-infiltrating lymphocytes and genetically engineered T cells with receptors targeted to HPV16 proteins.4
One such study is the phase 2 study C-145-04 (NCT03108495) that is evaluating the safety and efficacy of a nonmyeloablative lymphodepletion regimen followed by the autologous TIL therapy LN-145 and then IL-2 administration for the second-line treatment of patients with recurrent, metastatic, or persistent cervical carcinoma.34,35
Preliminary results from 27 patients who have at least 1 resectable lesion (diameter, 1.5 cm) and documented disease progression after the most recent therapy (mean prior therapies, 3) showed an ORR of 44.0% and DCR of 89.0% at the 3.5-month median follow-up.34,35
Antibody-Drug Conjugates
The approach behind the development of antibody-drug conjugates is to combine antibodies against antigens expressed by tumor cells with a cytotoxic agent, which offers the benefits of more targeted cytotoxicity and fewer systemic AEs.4,33
Many tumor cells express surface proteins that are typically not expressed in nonmalignant cells. These surface proteins can then be used as targets for antibody-drug conjugates.4 In normal physiological conditions, the cell-surface protein tissue factor (TF) is a key part of the coagulation cascade. However, in oncogenesis, TF is involved in cancer-related signaling pathways (eg, angiogenesis, tumor progression, and metastases), and TF expression is associated with poor clinical outcomes. TF is highly expressed in many solid tumors, including cervical cancer, making it an appealing therapeutic target.4,36
Tisotumab vedotin is an antibody-drug conjugate comprising a fully human monoclonal anti-TF antibody conjugated to the microtubule-disrupting agent monomethyl auristatin E. Its safety and efficacy were evaluated in the innovaTV 201 (NCT02001623) phase 1/2 dose escalation and expansion trial in patients with previously treated advanced solid tumors, including cervical cancer.4,36
Patients in the cervical cancer cohort received 2.0 mg/kg of tisotumab vedotin IV every 3 weeks for up to 12 cycles (if clinical benefit was noted after 4 cycles). Of the 55 patients enrolled into the cervical cancer expansion cohort, 73.0% had an ECOG PS of 1; 51.0% had SCC, 35.0% had ADC, and most had confirmed TF expression of at least 1.0%.36
The primary end point was safety. TRAE discontinuations occurred in 18.0% of patients, and 13.0% of patients required dose reductions because of TRAEs.35 The most common AEs were epistaxis (51.0%), fatigue (51.0%), nausea (49.0%), conjunctivitis (42.0%), and alopecia (40.0%). The rate of grade 3 or higher AEs was 53.0%; of these, the most common were vomiting (7.0%) and constipation (5.0%). AEs of special interest that occurred in more than 5% of patients were neuropathy events (55.0%), bleeding-related events (73.0%), and ocular events (65.0%).36
Among the 51 patients who received a prior treatment, the independent review committee-assessed confirmed ORR was 24.0%. The median DOR was 6.0 months and 6-month PFS was 40.0%.36
DNA Damage–Response Inhibitors
Radiation therapy acts primarily by damaging the DNA of irradiated cells.4 Damage to DNA activates a network of cellular DNA damage responses (DDRs), including DNA repair proteins and cell cycle regulators, that attempt to repair the lesions so cells can resume the cell cycle and growth. For the most part, tumor cells are less capable of repairing DNA than are nonmalignant cells, which is why radiation therapy can be an effective therapeutic approach for cancer. However, some tumor cells develop aberrant DDR expression (a fundamental characteristic of tumorigenesis), allowing them to become resistant to radiation therapy. Therefore, DDR inhibitors could help prevent tumor cells from being able to repair the DNA lesions caused by the radiation.4
Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) is an inhibitor of ribonucleotide reductase, a cell cycle regulator that is a member of the DDR network and that has well-characterized antiproliferative effects.4,37 It prolongs DNA repair time, arresting the cells at the G1/S checkpoint. The safety and efficacy of triapine in patients with advanced cervical or vaginal cancer were studied in a 6-week, phase 2 pilot study (NCT01835171), during which 13 patients were assigned to receive triapine (25 mg/m2) 3 times weekly in addition to cisplatin-based chemoradiotherapy. The other 13 patients in the study were given chemotherapy alone. Baseline patient characteristics were balanced across the 2 treatment groups; every patient received at least 1 treatment and was included in the safety analysis.37
The primary end point was the metabolic CR rate, which was higher among patients in the triapine group than among the chemotherapy group (92.0% vs 82.0%, respectively). The estimated 3-year PFS was higher among patients treated with triapine than chemotherapy alone (92.0% vs 77.0%).37
There were no significant differences regarding frequency of AEs between the 2 groups at the median follow-up of 40 months. The most common AEs reported were fatigue, nausea, diarrhea, electrolyte abnormalities, anemia, neutropenia, and leukopenia.37
A phase 3 trial (NCT02466971) is now underway to assess OS and PFS in patients treated with triapine.37
Conclusion
Cervical cancer is one of the most common gynecologic cancers, and advanced disease is associated with a high mortality worldwide.1-5 Immunotherapeutic approaches have shown promising results for treating advanced, metastatic, or recurrent cervical cancer and have focused on targeting HPV oncoproteins, mechanisms of immune evasion, and cancer-associated surface proteins.7,12,13
In clinical trials, various PD-1/PD-L1 inhibitors (eg, cemiplimab) have had a positive effect on survival with tolerable toxicity; approval of these agents will expand the treatment landscape for patients with cervical cancer.3,6,13,23,25
Other approaches with promising results in early clinical trials include adoptive T-cell therapies that induce deep and durable responses in a subset of patients, and antibody-drug conjugates that target cancer-associated surface proteins (eg, TF). This wide range of agents in the drug development pipeline suggests potential for more therapeutic choices and new opportunities to improve outcomes in patients with cervical cancer.4,35-37
REFERENCES
- Fowler JR, Maani EV, Jack BW. Cervical cancer. In: StatPearls. StatPearls Publishing. Updated July 7, 2021. Accessed September 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK431093/#_NBK431093_pubdet_
- Key statistics for cervical cancer. American Cancer Society. Updated January 12, 2021. Accessed September 20, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8599.00.pdf
- Cervical cancer treatment (PDQ)-health professional version. National Cancer Institute. Updated January 22, 2021. Accessed September 20, 2021. https://www.cancer.gov/types/cervical/hp/cervical-treatment-pdq#_388
- Randall LM, Walker AJ, Jia AY, Miller DT, Zamarin D. Expanding our impact in cervical cancer treatment: novel immunotherapies, radiation innovations, and consideration of rare histologies. Am Soc Clin Oncol Educ Book. 2021;41:252-263. doi:10.1200/EDBK_320411
- Yu L, Sabatino SA, White MC. Rural–urban and racial/ethnic disparities in invasive cervical cancer incidence in the United States, 2010–2014. Prev Chronic Dis. 2019;16:180447. doi:10.5888/pcd16.180447
- DeGroff A, Miller J, Sharma K, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January–June 2020, in the United States. Prev Med. 2021;151:106559. doi:10.1016/j.ypmed.2021.106559
- Liu Y, Wu L, Tong R, et al. PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol. 2019;10:65. doi:10.3389/fphar.2019.00065
- Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378-384. doi:10.1038/nature21386
- Feng YC, Ji WL, Yue N, Huang YC, Ma XM. The relationship between the PD-1/PD-L1 pathway and DNA mismatch repair in cervical cancer and its clinical significance. Cancer Manag Res. 2018;10:105-113. doi:10.2147/CMAR.S152232
- Meng Y, Liang H, Hu J, et al. PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer. 2018;9(16):2938-2945. doi:10.7150/jca.22532
- Enwere, EK, Kornaga EN, Dean M, et al. Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical cancer. Mod Pathol. 2017;30(4):577-586. doi:10.1038/modpathol.2016.221
- Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28(12):1594-1602. doi:10.1038/modpathol.2015.108
- Liu C, Lu J, Tian H, et al. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep. 2017;15(3):1063-1070. doi:10.3892/mmr.2017.6102
- Friedman CF, Charen AS, Zhou Q, et al. Phase II study of atezolizumab in combination with bevacizumab in patients with advanced cervical cancer. J Immunother Cancer. 2020;8(2):e001126. doi:10.1136/jitc-2020-001126
- Tewari KS, Monk BJ, Vergote I, et al. VP4-2021: EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9: interim analysis of phase III trial of cemiplimab vs. investigator’s choice (IC) chemotherapy (chemo) in recurrent/metastatic (R/M) cervical carcinoma.Ann Oncol. 2021;32(7):940-941. doi:10.1016/j.annonc.2021.04.009
- Tecentriq. Prescribing information. Genentech; 2021. Accessed September 23, 2021. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf
- Libtayo. Prescribing information. Regeneron Pharmaceuticals Inc. and Sanofi-Aventis US LLC; 2021. Accessed September 23, 2021. https://www.regeneron.com/downloads/libtayo_fpi.pdf
- Burova E, Hermann A, Waite J, et al. Characterization of the anti-PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16(5):861-870. doi:10.1158/1535-7163.MCT-16-0665
- Papadopoulos KP, Johnson ML, Lockhart AC, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 2020;26(5):1025-1033. doi:10.1158/1078-0432.CCR-19-2609
- Sternberg A. Cemiplimab impresses in a phase 3 trial of cervical cancer, regulatory submission expected. Cancer Network®. March 15, 2021. Accessed September 23, 2021. https://www.cancernetwork.com/view/cemiplimab-impresses-in-a-phase-3-trial-of-cervical-cancer-regulatory-submission-expected
- Study of cemiplimab in adults with cervical cancer. ClinicalTrials.gov. Updated July 15, 2020. Accessed September 23, 2021. https://clinicaltrials.gov/ct2/show/NCT03257267
- Opdivo. Prescribing information. Bristol-Myers Squibb; 2021. Accessed September 23, 2021. https://packageinserts.bms.com/pi/pi_opdivo.pdf
- Santin AD, Deng W, Frumovitz M, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol. 2020;157(1):161-166. doi:10.1016/j.ygyno.2019.12.034
- Naumann RW, Hollebecque A, Meyer T, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 Trial. J Clin Oncol. 2019;37(31):2825-2834. doi:10.1200/JCO.19.00739
- Imfinzi. Prescribing information. AstraZeneca; 2021. Accessed September 23, 2021.
https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/9496217c-08b3-432b-ab4f-538d795820bd/9496217c-08b3-432b-ab4f-538d795820bd_viewable_rendition__v.pdf - Mayadev J, Nunes AT, Li M, Marcovitz M, Lanasa MC, Monk BJ. CALLA: efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy vs chemoradiotherapy alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. Int J Gynecol Cancer. 2020;30(7):1065-1070. doi:10.1136/ijgc-2019-001135
- National Cancer Institute. Dictionary of cancer terms. Accessed November 23, 2021. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/stage-i-cervical-cancer
- Study of durvalumab with chemoradiotherapy for women with locally advanced cervical cancer (CALLA). ClinicalTrials.gov. Updated August 16, 2021. Accessed September 23, 2021. https://clinicaltrials.gov/ct2/show/record/NCT03830866
- Raedler LA. Keytruda (pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8(spec feature):96-100.
- Keytruda. Prescribing Information. Merck Sharp & Dohme Corp.; 2021. Accessed September 22, 2021. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. Published online September 18, 2021. doi:10.1056/NEJMoa2112435
- Zhu S, Zhang T, Zheng L, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14(1):156. doi:10.1186/s13045-021-01164-5
- Lindberg J, Nilvebrant J, Nygren PÅ, Lehmann F. Progress and future directions with peptide-drug conjugates for targeted cancer therapy. Molecules. 2021;26(19):6042. doi:10.3390/molecules26196042
- Study of LN-145, autologous tumor infiltrating lymphocytes in the treatment of patients with cervical carcinoma. ClinicalTrials.gov. Updated June 9, 2021. Accessed September 23, 2021. https://clinicaltrials.gov/ct2/show/NCT03108495
- Jazaeri AA, Zsiros E, Amaria RN, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2019;37(suppl 15):2538. doi:10.1200/JCO.2019.37.15_suppl.2538
- Hong DS, Concin N, Vergote I, et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin Cancer Res. 2020;26(6):1220-1228. doi:10.1158/1078-0432.CCR-19-2962
- Kunos CA, Andrews SJ, Moore KN, Chon HS, Ivy SP. Randomized phase II trial of triapine-cisplatin-radiotherapy for locally advanced stage uterine cervix or vaginal cancers. Front Oncol. 2019;9:1067. doi:10.3389/fonc.2019.01067

