- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Recent Developments in the Treatment of Advanced Basal Cell Carcinoma
Although relatively small in number, advanced basal cell carcinoma (BCC) cases often resist standard BCC therapy and require other types of treatment.1 Treating locally advanced BCC (laBCC) or metastatic BCC (mBCC) may involve a variety of treatment modalities, including surgery—standard excision, electrodessication and curettage, Mohs micrographic surgery, and/or resection with complete circumferential peripheral and deep margin assessment—radiation therapy (RT), targeted treatment with a Hedgehog pathway inhibitor (HHI), systemic immunotherapies, and/or participation in a clinical trial.2,3 Therefore, the involvement of surgical oncologists, radiation oncologists, and medical oncologists who specialize in each of these areas is key to achieving optimal outcomes for patients.4
When topical therapy, surgery, or RT is unlikely to be curative for recurrent or advanced BCC disease, systemic therapy may be considered.2 If systemic treatment is to be used, HHIs should be considered.2 Notably, HHIs are associated with an adverse event (AE) profile that results in treatment discontinuation in up to 30% of patients.4 In these cases, the multidisciplinary care team may consider dosage alterations and supportive care.4 For the many patients who initially experience a complete clinical response on an HHI but eventually become intolerant or experience tumor regrowth, second-line therapies such as immune checkpoint inhibitors (ICIs) may be considered.3,5
This article reviews the pathophysiology of advanced BCC and explores treatment options, focusing on HHIs and ICIs.
HHIs as First-Line Therapy
The HH signaling pathway is involved in the proper maintenance, renewal, and regeneration of tissues.3,6 Abnormal HH activity plays a role in the growth of many types of cancer.7 Relevant to BCC is that a gene known as PTCH1 codes for the HH receptor.6,8 Under normal circumstances, the PTCH1 protein coded by the PTCH1 gene inhibits the signaling activity of SMO, a transmembrane oncogenic protein.4,8 The SMO protein, in turn, signals for DNA replication within basal cells.6,8
The PTCH1 gene is highly susceptible to UV-induced damage from the sun’s rays.6 Mutated PTCH1 genes create versions of the PTCH1 protein that do not properly inhibit the SMO protein; this leaves the SMO in a constant state of activation, which leads to uncontrolled proliferation of basal cells.6,8 Aberrant activation of the HH signaling pathway, through a mutated PTCH1 gene or a mutated SMO gene, is responsible for nearly all cases of sporadic BCC, accounting for 85% and 10% of cases, respectively.3,9
HHIs are small molecules that restrain some element of the HH signaling pathway. Unlike traditional chemotherapy, which affects a wide range of cells, HHIs target specific molecules.1 Although HHIs targeting other components of the HH signaling pathway are being studied and have been developed for other cancers, those for advanced BCC have largely focused on inhibiting the SMO protein.3 The 2 HHIs currently approved for use in the advanced BCC setting are vismodegib and sonidegib.3,8,9
Vismodegib is approved for use in either laBCC or mBCC10; sonidegib currently has an indication for laBCC only.11 ERIVANCE (NCT00833417)8 and BOLT (NCT01327053)9 were the phase 2 trials that established the safety and efficacy of vismodegib and sonidegib in the advanced BCC setting, respectively.3,4,8,9
Vismodegib
The HHI vismodegib is a small molecule inhibitor of SMO.3,8 In ERIVANCE, the 96 patients (33 with mBCC and 63 with laBCC) who were ultimately included in the efficacy analysis were given 150 mg vismodegib once daily.8 The trial’s primary end point, as assessed by an independent review committee (IRC), was objective response rate (ORR).8 At the study’s data cutoff point—which came after a median duration of drug exposure of 10 months in the mBCC group and 9.7 months in the laBCC group8—ORR was experienced by 30% and 43% of patients, respectively.8 This was significantly higher than the predicted 10% (P = .001) and 20% (P < .001), respectively, expected for the null hypothesis in both groups.8
The safety analyses included 104 patients (33 with mBCC and 71 with laBCC).8 At the same data cutoff point and after the same median duration of drug exposure of approximately 10 months in both groups, the most common AE of any grade across both cohorts of patients included muscle spasms (68%), alopecia (63%), dysgeusia (51%), weight loss (46%), asthenia (36%), nausea (29%), loss of appetite (23%), and diarrhea (22%).8
Twelve percent (12%) experienced an AE leading to discontinuation of vismodegib; the most common reason was muscle spasms.8
Sonidegib
Sonidegib is also a small molecule SMO antagonist.3,9 In the BOLT trial, the 171 patients (36 with mBCC and 135 with laBCC) who were ultimately included in the efficacy analysis were randomized 1:2 to receive sonidegib 200 mg once daily (55 patients) or 800 mg once daily (116 patients).9 As in ERIVANCE, the primary end point in BOLT, as assessed by an IRC, was ORR.9 The median follow-up time among all patients included in the trial was 13.9 months.9 At follow-up, objective response in the 200-mg cohort was experienced by 15% of patients with mBCC and 47% of patients with laBCC, as well as by 17% of patients with mBCC and 35% of patients with laBCC in the 800-mg cohort.9
The safety analysis included 79 patients in the 200-mg dose cohort and 150 patients in the 800-mg dose cohort.9 After a median duration of drug exposure of 8.9 months in the 200-mg cohort and 6.5 months in the 800-mg cohort, there were numerically lower proportions of patients who experienced AEs of any grade in the 200-mg cohort than in the 800-mg cohort.9 The most common AEs were muscle spasms (49% [200-mg cohort] and 67% [800-mg cohort]), alopecia (43% and 55%), dysgeusia (38% and 59%), nausea (33% and 45%), increased serum creatinine (29% and 36%), fatigue (29% and 36%), weight loss (27% and 38%), and diarrhea (24% and 22%).9 Loss of appetite and myalgias were also commonly experienced in the 800-mg cohort.9
The rate of study drug discontinuation due to AEs was numerically lower in the 200-mg dose cohort compared with the 800-mg dose cohort (22% vs 36%, respectively), and the most common reason for study drug discontinuation in either cohort was muscle spasms.9 A numerically higher proportion of patients in the 200-mg dose group vs the 800-mg dose group were able to remain on therapy for 4 months or longer (91% vs 70%, respectively).9
The study investigators concluded that because antitumor activity was observed in both dose cohorts but the lower dose was associated with a more favorable AE profile, the 200-mg dose of sonidegib should be suggested for patients with advanced BCC.9
AE Mitigation Strategies and Ongoing HHI Challenges
The safety profile of HHIs is notable, with results from the ERIVANCE and BOLT trials indicating that AEs lead to treatment discontinuation in up to 30% of patients.4 Muscle spasms, dysgeusia, alopecia, asthenia, and weight loss are common among the HHIs as a class and are experienced by the majority of patients.4,12 In addition, vismodegib and sonidegib have a moderate to high emetogenic potential (ie, causes nausea and vomiting).12
Strategies utilized to attenuate toxicities and improve drug tolerability and quality of life (QOL) include intermittent dosing schedules (treatment interruptions [drug holidays] or every-other-day dosing), dose reductions, and/or supportive care with pharmacological agents.3,4,12
Calcium channel blockers (eg, amlodipine, diltiazem, or verapamil), nerve stabilizers (eg, gabapentin, pregabalin), muscle relaxers (eg, cyclobenzaprine), lidocaine, levetiracetam, and vitamin B complex have demonstrated effectiveness in treating HHI-induced muscle spasms of grade 1/2.12 For muscle spasms of grade 3 and higher, a treatment interruption for at least 2 cycles or changing to a 1-week-on/1-week-off dosing regimen have proved to be helpful.12
A variety of dietary modifications (eg, adding spice, lemons, or sweeteners) can be recommended to help mitigate the taste dampening associated with HHIs.12 A dose interruption of at least 4 weeks is usually required for taste sensation to normalize, considering the half-life of HHIs and the life span of taste cells (10-24 days).12
Use of minoxidil and dihydrotestosterone inhibitors (eg, spironolactone, finasteride) can be recommended for HHI-induced alopecia.12 However, it is important for patients to understand that the HHI-induced alopecia is different in nature from chemotherapy-induced alopecia, in that it develops gradually, could be delayed, and can occur after HHI therapy is stopped.12
Use of levocarnitine and delta-9-tetrahydrocannabinol (ie, medical marijuana) may also be useful for treating muscle spasms and taste disturbances associated with HHI therapy, respectively.12
Although the adjunctive care used to mitigate HHI AEs can increase patient QOL,4 some challenges remain regarding tolerability and safety. For example, some patients who initially experience a complete clinical response on an HHI eventually experience tumor regrowth,3,5 making the optimal duration of treatment hard to define and overall treatment costs hard to predict. Other patients become intolerant to HHI therapy.3,5 On account of regrowth or intolerance, many patients discontinue treatment entirely.5,8,9 For instance, in the final analysis of the ERIVANCE trial, which included 39 months of follow-up data, only 8 of 104 patients (7.7%) were continuing to receive treatment with vismodegib per protocol, whereas the vast majority (n = 96; 92.3%) had discontinued treatment. The most common reasons for discontinuation were AEs (21.2%) or disease progression (27.9%).5
ICIs and Emerging Options in Treating Advanced BCC
For reasons of efficacy, QOL, and overall disease-management costs, patients who progress on HHI therapy or still cannot tolerate the AEs often require a second-line option. In February 2021, the ICI cemiplimab was approved by the FDA for use in patients with laBCC who have been previously treated with an HHI or for whom an HHI is not appropriate.13,14 Prior to this approval, patients with laBCC had no approved therapeutic option beyond first-line treatment with an HHI.14 The FDA placed cemiplimab under accelerated approval for use in mBCC as well. Other HHIs, other ICIs, and the antifungal agent itraconazole are also being investigated to treat advanced BCC.
Cemiplimab
Cemiplimab gained its first US approval in 2018 for use in locally advanced or metastatic cutaneous squamous cell carcinoma. Like other ICIs, and unlike HHIs, cemiplimab seeks to restimulate the immune system after cancer has suppressed it and to promote T-cell activity and cancer surveillance.13,15-18 Cemiplimab targets PD-L1 and helps prevent PD-L1 from binding to and activating the PD-1 receptor on the surface of T cells. Therefore, this fully human monoclonal antibody (mAB) blocks cancer cells from suppressing T-cell activation.13,15,19
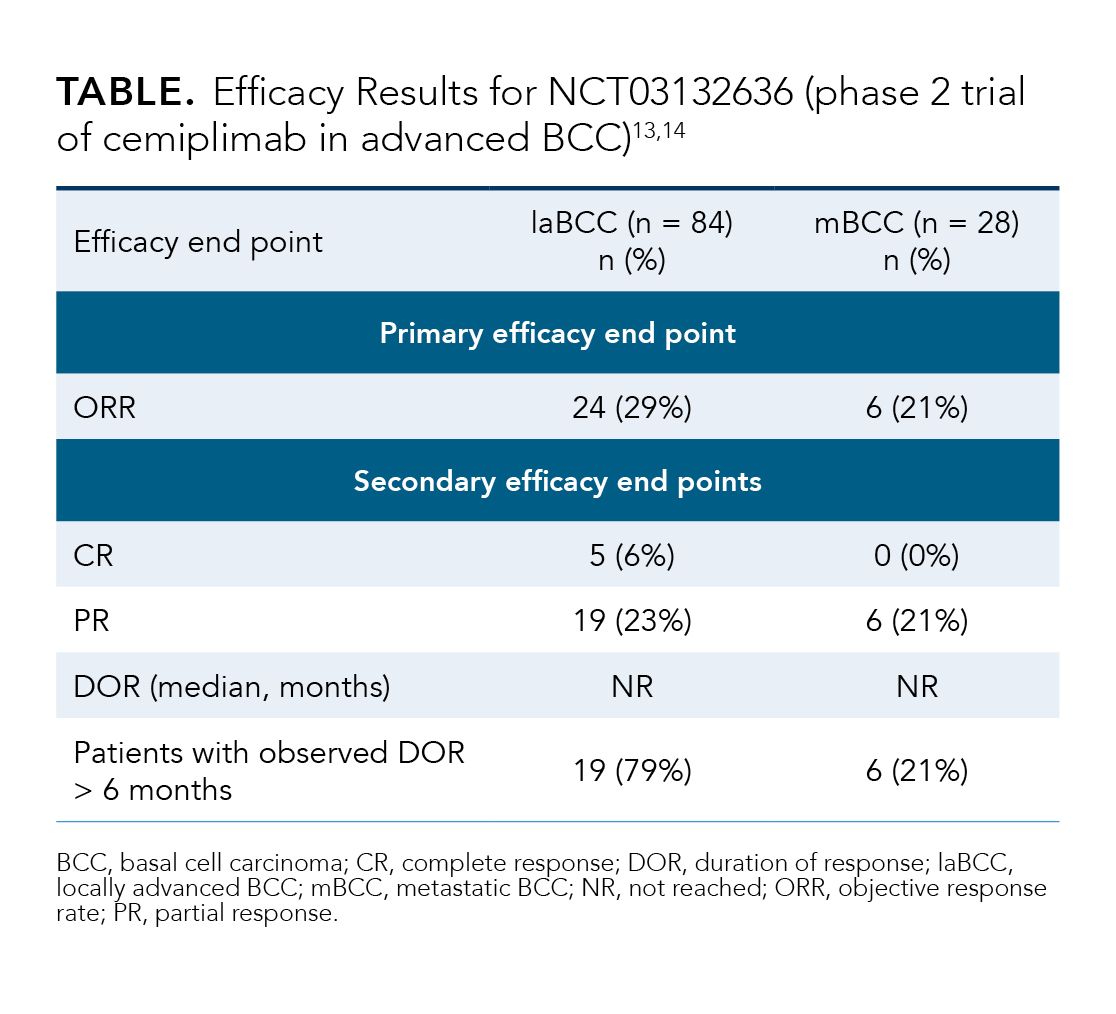
The approval of cemiplimab was based on the results of Study 1620 (NCT03132636), an open-label, multicenter, nonrandomized, single-arm phase 2 study.13,14 In the trial, investigators evaluated the efficacy and safety of cemiplimab 350 mg every 3 weeks in patients with laBCC or mBCC who had previously been treated with an HHI.13,14 Patients who had progressed on, failed (did not achieve an objective response after 9 months), or were intolerant to HHI therapy were included in the trial.13,14 In addition, patients had to be ineligible for curative surgery or curative RT.14 The primary efficacy end point was ORR, as assessed by IRC.14
The Study 1620 results are shown in the Table.13,14 Among the efficacy population (n = 121), the ORRs were 29% and 21% for the laBCC and mBCC cohorts, respectively.13,14
After a median duration of cemiplimab exposure of 42 weeks in the safety population (n = 132),13 the most common AEs of any grade werefatigue (49%), musculoskeletal pain (33%), diarrhea (25%), rash (22%), pruritus (20%), and upper respiratory tract infection (15%).13 Discontinuation of cemiplimab during the trial because of AEs occurred in 13% of patients, with the most common reasons being colitis and overall physical health deterioration.13
Study 1620 demonstrated the efficacy of cemiplimab in treating laBCC in patients who had previously received HHI therapy.14 Cemiplimab shows promise for the many patients who discontinue HHI therapy because of toxicity or disease progression.14 Moreover, it ushers in the entire ICI class and opens a new horizon for advanced BCC therapy.
Investigational Agents
Beyond the approval of cemiplimab, the horizon for immune approaches to advanced BCC appears promising. Several HHIs—glasdegib (PF-04449913), taladegib (LY2940680), BMS-833923—along with the antifungal agent itraconazole are being researched as advanced BCC therapies.3,6,20 Moreover, certain ICIs that have been approved for use in other cancers are being investigated for use in BCC. These include pembrolizumab16,17 and nivolumab with or without ipilimumab.18,19,21 Like cemiplimab, pembrolizumab and nivolumab are both mABs that block the PD-1 receptor on T cells.17,18 Ipilimumab is a mAB that binds to CTLA-4, an inhibitor of T-cell activity.21 Blockade of CTLA-4 allows effector T cells to activate, proliferate, and infiltrate tumors instead of remaining in a quiescent state.21
Pembrolizumab is being studied for advanced BCC treatment both as a monotherapy and in combination with an HHI. In a proof-of-principle, nonrandomized, open-label study (N = 16) of pembrolizumab with or without vismodegib given to patients with advanced BCC, the ORR was 38% at 18 weeks (44% for the pembrolizumab monotherapy group and 29% for the dual-therapy group).19 Among the 6 patients who demonstrated a response, the median duration of response (DOR) was 67.3 weeks.19 Progression-free survival (PFS) and overall survival (OS) at 1 year were 70% and 94%, respectively.19 Of a total of 98 AEs observed (any grade), none were life-threatening, and only 3 were considered severe (grade 3).19
Nivolumab with or without ipilimumab is being studied for use in laBCC and mBCC in an ongoing phase 2 open-label trial (NCT03521830).22 Nivolumab monotherapy is being given to patients who are PD-1 inhibitor naive and have unresectable disease previously treated with 2 or fewer prior systemic therapies (cohort A).22 Nivolumab plus ipilimumab dual therapy is being given to patients with disease progression after being on PD-1 inhibitor therapy (cohort B).22 The primary outcome measure is ORR; secondary outcome measures are PFS, DOR, and OS. 22
Conclusions
Given the challenges of treating advanced BCCs, the approval of a new class of agents coupled with a robust pipeline suggests the potential for improved outcomes for patients. With more avenues for interventions and a continued emphasis on multidisciplinary approaches, healthcare professionals may also learn more about the risks and likelihoods of BCCs becoming advanced and identify appropriate treatments for the optimization of patient care.
References
- Zelin E, Zalaudek I, Agozzino M, et al. Neoadjuvant therapy for non-melanoma skin cancer: updated therapeutic approaches for basal, squamous, and Merkel cell carcinoma. Curr Treat Options Oncol. 2021;22(4):35. doi:10.1007/s11864-021-00826-3
- NCCN. Clinical Practice Guidelines in Oncology. Basal cell skin cancer, version 2.2021. Accessed May 6, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1416
- Migden MR, Chang ALS, Dirix L, Stratigos AJ, Lear JT. Emerging trends in the treatment of advanced basal cell carcinoma. Cancer Treat Rev. 2018;64:1-10. doi:10.1016/j.ctrv.2017.12.009
- Peris K, Fargnoli MC, Garbe C, et al; European Dermatology Forum (EDF); the European Association of Dermato-Oncology (EADO); European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17(1):332. doi:10.1186/s12885-017-3286-5.
- Harris L. Basal cell carcinoma: a pharmacist’s guide. US Pharm. 2019;44(8):29-35. Accessed June 21, 2021. https://www.uspharmacist.com/article/basal-cell-carcinoma-a-pharmacists-guide
- Chaudhry P, Singh M, Triche TJ, Guzman M, Merchant AA. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood. 2017;129(26):3465-3475. doi:10.1182/blood-2016-05-718585
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171-2179. doi:10.1056/NEJMoa1113713
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716-728. doi:10.1016/S1470-2045(15)70100-2
- Vismodegib. Prescribing information. Genentech; 2020. Accessed May 6, 2021. https://www.gene.com/download/pdf/erivedge_prescribing.pdf
- Sonidegib. Prescribing information. Sun Pharma; 2019. Accessed May 6, 2021. https://www.odomzo.com/themes/custom/odomzo/global/pdfs/pi.pdf
- Lacouture ME, Dréno B, Ascierto PA, et al. Characterization and management of hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21(10):1218-1229. doi:10.1634/theoncologist.2016-0186
- Cemiplimab. Prescribing information. Regeneron; 2021. Accessed May 13, 2021. https://www.regeneron.com/downloads/libtayo_fpi.pdf
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848-857. doi:10.1016/S1470-2045(21)00126-1
- Walter A, Barysch MJ, Behnke S, et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin Cancer Res. 2010;16(14):3562-3570. doi:10.1158/1078-0432.CCR-09-3136
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20(4):477-482. doi:10.1007/s40257-019-00426-w
- Pembrolizumab. Prescribing information. Merck; 2021. Accessed May 17, 2021. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- Nivolumab. Prescribing information. Bristol-Myers Squibb; 2021. Accessed May 17, 2021. https://packageinserts.bms.com/pi/pi_opdivo.pdf
- Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: an investigator-initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80(2):564-566. doi:10.1016/j.jaad.2018.08.017
- Sabu DM, Kroes J, Gilham C, et al. Neoadjuvant vismodegib followed by radiation in locally advanced basal cell carcinoma. Current Problems in Cancer. Published online April 1, 2021. doi:10.1016/j.currproblcancer.2021.100736
- Ipilimumab. Prescribing information. Bristol-Myers Squibb; 2020. Accessed May 17, 2021. https://packageinserts.bms.com/pi/pi_yervoy.pdf
- Nivolumab alone or plus relatlimab or ipilimumab for patients with locally-advanced unresectable or metastatic basal cell carcinoma. ClinicalTrials.gov. Updated March 8, 2021. Accessed May 13, 2021. https://clinicaltrials.gov/ct2/show/NCT03521830

