- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Real-World Data Support Selexipag in the Treatment of PAH
Researchers used real-world data to further validate the use of selexipag in the management of pulmonary arterial hypertension (PAH).
Researchers have confirmed the efficacy and benefits of selexipag in the treatment of patients with pulmonary arterial hypertension (PAH), according to a study published in The Journal of Heart and Lung Transplantation.
Selexipag is an approved oral treatment for the management of PAH. To date, it has demonstrated the ability to hinder risks of PAH-related hospitalization and slow disease progression. These data are backed up by the GRIPHON trial, which correlated selexipag with a 40% reduction in disease progression when integrated as a monotherapy, dual therapy, or triple therapy.
The authors of the current study point out the increasing value of real-world evidence that can be used to bolster findings from clinical trials. Particularly, they note how the diversity of real-world studies better reflects affected patient populations compared with trial cohorts, which may be more limited.
PAH Model | image credit: Alila Medical Media - stock.adobe.com
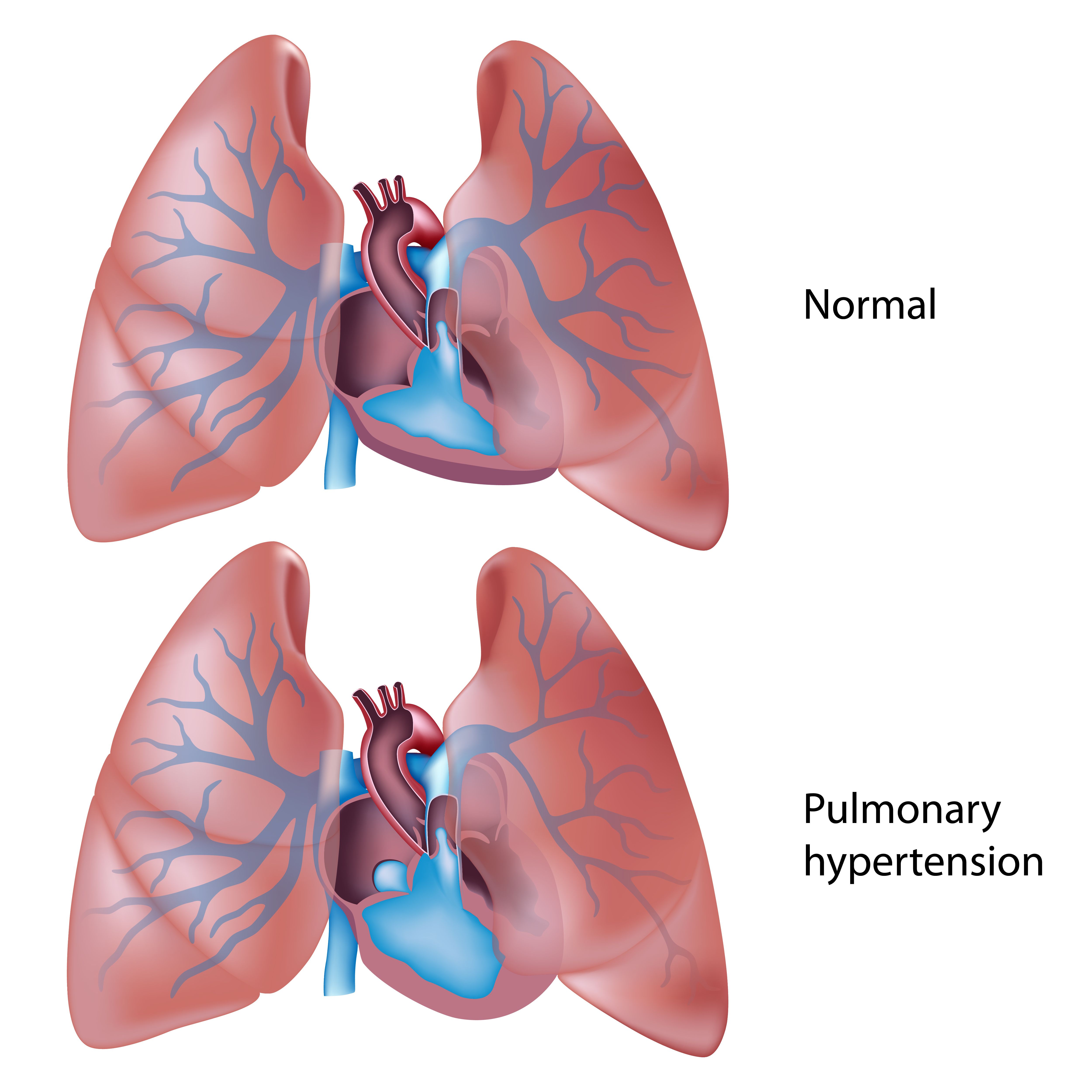
To complement the GRIPHON trial, patients were enrolled from SelexiPag: tHe usErs dRug rEgistry (SPHERE) between November 2016 and March 2020. This database is a US-based, multicentered, prospective, observational, real-world registry of patients administered selexipag. Eligible patients were categorized as “newly initiated” if they began treatment with selexipag within 60 days of the study period, and “previously initiated” if they began more than 60 days before the study.
Of the 829 patients enrolled from SPHERE, 759 were identified with PAH. In this group, 51.9% were newly initiated with selexipag and 48.1% were previously initiated. Patients’ baseline disease severities and progression were assessed according to their World Health Organization functional class (FC) and 1-year mortality risks were calculated using the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL 2.0, which characterizes individuals as at low, intermediate, or high risk). Patients were followed for up to 18 months and data were recorded from their routine clinical visits.
At baseline, over 50% of patients were FC class III. In the total group, 42.7%, 30.2%, and 27.1% were initially assessed as low, intermediate, and high risk, respectively. Additionally, 114 patients (15%) received selexipag twice daily at a dosage of 200 to 400 μg, 238 patients (31.4%) at 600 to 1000 μg, and 310 (40.8%) received more than 1200 μg. The remaining individuals either had a different dosage or their titration went unrecorded.
For most patients, a stable or improved FC status was observed (68.5% and 20.2%, respectively, to month 6; 65.6% and 22.0% to month 12; and 61.1% and 24.9% to month 18). These trends were also observed with REVEAL 2.0 calculations (62.9% and 22.1%, respectively, to month 6; 59.7% and 19.6% to month 12; and 57.2% and 21.3% to month 18). These results were similar for both the newly and previously initiated groups.
Overall survival at 12 and 18 months totaled at rates of 93.4% and 89.4%. These rates were high between each selexipag group; however, newly initiated patients demonstrated poorer survival rates compared with those previously initiated (83.8% vs 94.6%). The authors also noted that those whose REVEAL 2.0 calculations assessed them as intermediate or high risk had worse survival outcomes than low-risk individuals. Intermediate- and high-risk individuals also demonstrated a higher likelihood of hospitalization and experiencing adverse events.
The authors’ findings further validated the results of the GRIPHON trial by indicating that selexipag intervention contributes to delayed PAH progression and improved patient outcomes. In their concluding thoughts, they highlighted how—despite late initiation of treatment—results from SPHERE demonstrate how earlier therapy interventions can increase the potential for better management of PAH and outcomes in this patient population.
Reference
McLaughlin V, Farber H, Highland KB, et al. Disease characteristics, treatments, and outcomes of patients with pulmonary arterial hypertension treated with selexipag in real-world settings from the SPHERE registry (SelexiPag: tHe usErs dRug rEgistry). Heart Lung Transplant. 2023:S1053-2498(23)02027-2. doi:10.1016/j.healun.2023.09.016
