- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Rare Disease: Access, Reimbursement, and Disease Management A Stakeholder Interchange Report
On November 11, 2021, a group of health care decision makers convened for a virtual Scientific Interchange sponsored by the American Journal of Managed Care®. The event, Rare Disease: Access, Reimbursement, and Disease Management, was co-moderated by Neil Minkoff, MD, chief medical officer of Coeus Consulting Group in Sudbury, Massachusetts; and Sami Khella, MD, chief of the Department of Neurology at Penn Presbyterian Medical Center and professor at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia. Eight faculty participated: Eric Cannon, PharmD, FAMCP, chief pharmacy benefits officer of SelectHealth, Intermountain Healthcare in Salt Lake City, Utah; Dana McCormick, RPh, director of pharmacy at Blue Cross Blue Shield of Texas in Richardson, Texas; Tracey Davis, PharmD, director of pharmacy at AmeriHealth Caritas DC in Washington, DC; Kevin U. Stephens Sr, MD, JD, regional chief medical officer for employer and individual accounts with UnitedHealth Group, in New Orleans, Louisiana; Steve Evans, MD, vice president of quality and utilization management at Intermountain Healthcare in Las Vegas, Nevada; Jovonne Williams, PharmD, BCACP, clinical pharmacist in home infusion at Optum in Columbia, Maryland; Estay Greene, PharmD, MBA, vice president of pharmacy at Zing Health in Chicago, Illinois; and Stephanie Spence, PharmD, program director of specialty pharmacy at CareFirst Blue Cross Blue Shield in Baltimore, Maryland. In their discussion, they explored utilization management tactics, drug benefit design, and implications of site of care on patient access within the rare disease space, all from the perspectives of both provider and payer. Additionally, panelists discussed considerations to assess potential practices and models to improve and enhance delivery and access to care in rare disease, using hereditary amyloid transthyretin (hATTR) amyloidosis and hemophagocytic lymphohistiocytosis (HLH) as examples of chronic and acute rare diseases.
RARE DISEASES
Approximately 25 million to 30 million people in the United States are estimated to have a rare disease, defined as any disease that affects less than 200,000 Americans.1,2 About 7000 rare diseases have been identified. Although tremendous breakthroughs have occurred in the management of rare disease, approved treatments are available for less than 15% of these diseases.1
hATTR Amyloidosis
hATTR amyloidosis is a rare, multisystem, progressive disease that affects approximately 50,000 people worldwide.3,4 Transthyretin (TTR), a protein made primarily in the liver, normally exists as a tetramer, but in amyloidosis, it disassociates to monomeric entities that misfold and form into insoluble amyloid fibrils. hATTR is characterized by deposits of these fibrils in the nervous system, soft tissues, and organs.4-6 Accumulation of these fibrils leads to tissue toxicity and damage over time. hATTR is debilitating and ultimately fatal.3,5 Symptoms associated with hATTR include ocular disturbances, cardiac abnormalities, gastrointestinal issues, carpal tunnel syndrome, autonomic neuropathy, and peripheral neuropathy.6
The timing of hATTR disease presentation varies tremendously, with patients developing symptoms in their second to ninth decade of life.5 Although hATTR is a multisystem disease, dominant features are polyneuropathy and cardiomyopathy.5 Khella expressed that patients with polyneuropathy can experience significant weakness, pain, and diarrhea that can be socially disabling. Time from hATTR onset to death is approximately 10 years, with differences observed based on several factors, including geography, genotype, and symptoms.5
Approximately 150 variants of genes have been associated with development of hATTR.7 The impact of genetic variation on mortality was demonstrated in the Transthyretin Amyloidosis Cardiac Study among patients with cardiomyopathy. Compared with those having wild-type disease, patients with the TTR Val122Ilemutation had significantly shorter median overall survival time (43.0 vs 25.6 months; P = .04).8
Diagnosis of hATTR can be a challenge, and numerous specialists may diagnose and treat the disease, including neurologists, cardiologists, gastroenterologists, and ophthalmologists, among others. Management of hATTR often requires a multidisciplinary approach.5 Genetic testing and a positive result from an amyloid biopsy are appropriate to confirm a diagnosis of hATTR.5
Early identification of hATTR is beneficial to improve outcomes.5 Management goals in hATTR include reducing symptoms and severity and establishing a long-term treatment plan.5 Prior management of hATTR relied on symptomatic treatment and ultimately included liver transplantation.5 According to Khella, current treatments for the disease focus on stabilizing the TTR tetramer, suppressing the formation of the protein, and/or removing the amyloid deposits in the tissue.
Two agents that suppress ribonucleic acid from forming the TTR protein are patisiran and inotersen. Patisiran, an intravenous injection, was approved by the FDA in 2018 for the treatment of adults with hATTR amyloidosis–related polyneuropathy.9 Inotersen was also approved by the FDA in 2018 for the treatment of hATTR amyloidosis–related polyneuropathy in adults; however, it is a subcutaneous formulation.10 Another agent, tafamidis, is an oral selective stabilizer of the TTR tetramer. It was approved by the FDA in 2019 and is indicated for the treatment of the cardiomyopathy of wild-type or hATTR amyloidosis in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization.11 Several other agents are under investigation or in development that target various stages in the pathogenesis of ATTR amyloidosis.12
HLH
HLH is a rare, often fatal disease characterized by uncontrolled immune activation.13 While hATTR is a progressive, chronic disorder, HLH is an acute disease. The highest incidence occurs in patients aged less than 3 months, affecting boys and girls almost equally.13 The epidemiology is anywhere from 1 to 225 per 300,000 live births.13
HLH has 2 forms: primary and secondary.14 The primary autosomal recessive form, which represents about a quarter of all HLH cases, is known as familial hemophagocytic lymphohistiocytosis (FHL). FHL is associated with a median survival of less than 2 months after diagnosis if untreated, and it typically presents in the first year of life.14 Secondary HLH, also known as acquired HLH, may develop as a result of a severe infection or malignancy.14
Common symptoms of HLH are fever, hepatosplenomegaly, and cytopenias.14 Hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis, hyperferritinemia, impaired natural killer cell function, and elevated soluble CD25 may also occur.14 One set of diagnostic and therapeutic guidelines for HLH, called HLH-2004, indicates that 5 of these 8 criteria must be present for a diagnosis of HLH.14
HLH is difficult to diagnose and manage.14 Although molecular diagnosis has improved, it can be difficult to distinguish familial and secondary forms of HLH.14 HLH-2004 as well as another treatment protocol, HLH-94, were designed to diagnose, classify, and treat patients with HLH aged less than 18 years.15 For adults, individualized dosing and duration of treatments used in the HLH-94 protocol are recommended and are based on controlling the underlying trigger. Patients with a malignancy or infection should receive disease-specific treatment.15
The HLH-94 protocol consists of weekly dexamethasone and etoposide therapy.15 Patients with central nervous system (CNS) disease should receive intrathecal methotrexate and corticosteroids.14,15 If no improvement is observed after induction therapy, treatment should be continued until allogeneic hematopoietic cell transplantation.15 In the HLH-2004 treatment protocol, cyclosporine A was given during the induction phase. Although this reduced pretransplant mortality, albeit not significantly, safety and tolerability considerations maintain HLH-94 as the standard of care.15
Emapalumab-lzsg, a monoclonal antibody given as an intravenous injection, is the first and only agent available to treat primary HLH.16 It was approved by the FDA in 2018 for the treatment of adult and pediatric (newborn and older) patients with primary HLH with refractory, recurrent, or progressive disease or intolerance to conventional HLH therapy.16 Emapalumab-lzsg neutralizes interferon gamma; hypersecretion of interferon gamma has been implicated in the pathogenesis of HLH.16
In a multicenter, open-label trial with 27 pediatric patients with primary HLH (refractory, recurrent, or progressive disease during conventional HLH therapy or intolerance to conventional HLH therapy), the overall response rate to emapalumab-lzsg at the end of treatment was 63% (based on improvements in fever, splenomegaly, CNS symptoms, complete blood count, fibrinogen and/or D-dimer, ferritin, and soluble CD25 levels).16
The Evolution and Potential Impact of New Therapies for Rare Diseases
Minkoff and Khella stated that hATTR and HLH represent considerably underdiagnosed rare diseases. And notably, the number of rare diseases is quickly increasing due to improved diagnostics.17 Fortunately, Minkoff acknowledged that within the managed care space on both the provider and payer sides, the understanding and “explosion” of therapies for rare diseases has become a “huge part of management.”According to Minkoff, “In 2020, for the first time, specialty drugs overshadowed traditional drugs in terms of overall cost.” While an increasing number of “first-in-class” medications exist for rare diseases, the medical community has some reservations concerning their use due to knowledge gaps about their mechanisms of action.17 These specialized therapies also have major financial implications for patients and payers. As Cannon stated, “With these rare disease states, with these expensive treatments, it will become harder and harder for smaller [health] plans, smaller regional plans, to survive.”
UNDERSTANDING PRIORITIES AND UNMET NEEDS FOR RARE DISEASE MANAGEMENT ACROSS DIFFERENT SITES OF CARE
Priorities for Rare Disease Management
According to Stephens, patients with rare disease “are very, very complicated and complex, and it takes a whole system of care [to treat them properly]. Because it’s already problematic that they have a rare illness,…we try to throw what we can at them to make sure that they have the best experience possible.” To manage such patients’ disease, said Stephens, plans focus on (1) the total number of patients or total financial impact, and (2) the whole-patient experience, which includes wraparound services.
The value of therapies for rare disease is difficult to assess, and priorities for rare disease management differ between acute and chronic rare diseases. In chronic diseases, cost management strategies focus on policies outside of the benefit, such as stop-loss or reinsurance. According to Cannon, less time is spent discussing how to control the expense, but he questions whether managed care organizations have appropriate policies in place, such as reinsurance, and whether [or not they are] sufficient.
For acute therapies, plans look at the probability of a claim based on population characteristics, and they seek ways to mitigate the cost of a 1-time claim. Said McCormick, “I feel like we spend a fair amount of time kind of defending why we’re covering [new orphan drugs], and … that providing a pharmaceutical option offset[s] overall medical expenses.” Evans indicated that he is “not trying to be cost-efficient in the sense of limiting care. I’m trying to be cost-efficient in terms of making sure they have the best outcomes.”
Effective Care for Patients With Rare Diseases
It is critical for patients with rare diseases to receive appropriate treatment at the right time. Davis said, “It’s just one of those situations where if it’s medically necessary, as a payer in the Medicaid space, we’re going to cover it. And that’s why we are so tight on other areas where we can find cost savings.” Payers are also tasked with the job of determining how patients can receive appropriate coverage. “[We] focus on getting their access to care, [ensuring that] their caregivers [are] available, [finding out] where they’re going to be treated, as well as ensuring that we’re using the right payer system,” said Williams. “Sometimes it goes through the pharmacy benefit manager, but sometimes we can use the medical benefits in order to optimize their therapy. If they have been diagnosed, and we know the correct medication to use, and it’s available to them, I think there is opportunity to maximize their management by utilizing other forms of payment.... Overall, I haven’t seen as much limitation in them getting their medications, if there’s a clinical need for it. It’s just coming down to being able to provide them what they need, based on who will be able to pay for it for them.” When needed, billing through the medical benefit rather than the pharmacy benefit provides leverage for providers to ensure that a patient’s medication is covered based on where the medication is administered (eg, home, hospital, infusion center, physician’s office).
However, Stephens indicated that sometimes it is difficult to rationalize costs, and the input of medical specialists is important. “If the drug measurement is experimental, investigational, [or the] data [are] ‘loose,’ particularly in very rare illness and disease, [I] would highly suggest utilizing the peer-to-peer format,” Stephens said.McCormick echoed his thoughts, adding, “It’s a hard decision to make when you’ve got 10 patients in a study that drives an FDA indication. I feel like there’s a little more sensitivity and focus on what data [have] been provided to drive an indication, and I sense more restrictions and more potential noncoverage for medical necessity for some products that are coming out with limited data as a part of their study designs.”Other participants agreed that it is difficult to make informed decisions among rare disease agents, particularly when sample sizes in clinical trials are small, data are limited, and outcomes and the impact of therapy are difficult to assess.
Geographical Influence of Care for Patients With Rare Diseases
For patients with a rare disease, particularly for those living in rural areas, it can be difficult to gain access to a specialist, sometimes at an academic medical center, who can appropriately diagnose and manage their disease early in its course. Williams acknowledged that she sees patients who “travel—based on feedback, based on their connections—to try to find these specialist doctors, [ones] who have the [right] network and experience, [who can] diagnose [the disease early enough]. We see an uptick in certain providers being able to identify these rare disease states.” Williams said that once a patient is diagnosed with a rare disease, she tries to ensure that they can continue to access care and receive the medications they need. Cannon added that his colleagues and he have “work[ed] very closely with … individuals in terms of level-setting expectations, and then the next piece is really making sure we’re handling the transportation or making sure the family has the ability to go where they may want to go.” Williams acknowledged that “being in the home infusion space [has] definitely been an opportunity to try to help with being able to get families access, [especially] when you’re thinking about [the] elderly, [pediatric patients], and people who need caregivers. [We] try to get the nurses to the patients so that they’re not leaving their home.” Although telehealth can help ease the travel burden for some patients, some providers may not offer this service due to lower reimbursement compared with office visits.
Payer’s Role in Supporting Identification of Rare Diseases
Minkoff asked the roundtable participants to describe how their organizations are playing a role in identifying patients with rare diseases, such as the use of claims-based data or utilization patterns. According to McCormick, whether or how a payer is involved in patient identification depends on the payer type. “An integrated delivery network where the payer and the physicians are more closely aligned [may] … make [the most] sense,” she said. Her organization “operates more as a collaboration. I feel like, as a national payer, our relationships with our providers are not situated such that we have that kind of relationship.”However, Cannon expressed that “as a payer that has a very tight relationship with the delivery system, I don’t see us, as an organization, aggressively going out and looking for rare and ultrarare disease states. I think we clearly have facilities to take care of these patients, but we also are trying to focus on delivering the best health care possible to the entire population.”
In identifying patients with rare diseases, payers typically focus on high-cost claimants or patients with increased utilization; they are not involved with diagnosis, since diagnosing carries the potential liability of underdiagnosed or misdiagnosed patients. “As the payer, it makes sense for us to pay attention to who is consistently going to the hospital, who’s consistently going to the emergency department [ED],” said Davis. “We want to keep our patients healthy, so we look for high-cost patients, and we review them every month.” Stephens agreed, adding that his colleagues and he “don’t just arbitrarily mine our data for rare diseases and illnesses. What happens is that you have someone who has increased utilization for whatever reason it may be—recurrent ED visit[s], recurrent admissions—that will give us a red flag. Then we try to catch [problems] early in the process when they are potentially misdiagnosed and maybe have a rare disease that we, frankly, just miss, and then we can participate with them.” Spence echoed this sentiment, but she added that in the case of hATTR, “some of the symptoms of this diagnosis will intersect with disease states that are in scope for quality measures, [like] … neuropathy and the use of opioids or other drugs. Payers may become more aware of patients who are using those therapies. I think by way of that initiative, I [could] identify these patients almost [by] accident, so I think there’s a role, but perhaps not a prescriptive role for payers.”
Engagement of Patients With Rare Diseases to Support Patient-Centered Care
Although payers do not have a strong proactive role in the identification of patients with rare diseases, once a diagnosis is confirmed, roundtable participants indicated that they focus on patient-centered care to support better outcomes. As McCormick expressed, “Once we do identify people, we do engage with a fully human-centered approach.”
Rare diseases, such as hATTR, are very difficult to manage, and patients and their families need social and moral support.5 Davis communicated that her clinical team goes through their cases and determines if they can offer any other support. Patients who are diagnosed with a rare disease need assistance in navigating the complex system. Collaboration between the care managers for the health plan and health system is necessary to provide effective patient-centered care. Said Cannon, “At the point in time when we know about that patient with rare disease, there’s real collaboration that goes on, because we have care management on the health system side, and we have care management on the health plan side. We don’t want those 2 to go down different paths. We try to coordinate that, to work in concert with each other on what is the best management.”
HEALTH CARE DECISION-MAKING OPPORTUNITIES AND CHALLENGES IN ACUTE AND CHRONIC RARE DISEASES
As stated earlier, the diagnosis of some rare diseases, particularly hATTR, which is chronic, can be extremely difficult and time-consuming. According to Khella, his challenge is seeing patients early enough in the course of their disease. Approximately a quarter of patients diagnosed with hATTR, he said, “have seen 5 physicians, and it’s taken, on average, 3 years to get a diagnosis.”18 Although the American Heart Association recently published guidelines for the management of cardiac amyloidosis,19 the lack of guidelines for treating patients with hATTR in general creates a knowledge gap and confusion regarding best management practices.12 In the case of the acute rare disease HLH, recommendations have been published fairly recently that outline management options.15
Payer Perspective
Payers participating in the roundtable indicated that is it easier to justify the use of a high-cost agent if it targets a specific genetic marker associated with a rare disease. Specifically, “it’s much easier to explain the cost of a product or the importance of a product if specific genetic links and genetic markers are associated with that product,” said Cannon. The presence of a genetic marker for a rare disease also informs health care decision makers about the need to screen family members.
Payers see value in pharmaceutical companies providing education on the identification of rare diseases and patient support. “They’ve done a really good job at trying to promote education [around hATTR] … to be able to have a neurologist diagnose these patients early,” noted Khella. He further explained that pharmaceutical companies “have made a concerted effort to educate providers as to when [they] should … be thinking about this disease when [they] see a patient with a neuropathy. They have gone [to] national meetings … [and] educat[ed] the sales rep[resentatives], who can then leave [lists of] the ‘red flag’ symptoms with the providers. They definitely have a role, because obviously they have a very vested interest in finding these patients.” Williams echoed that there may be “an opportunity … for pharmaceutical companies to assist with trying to help identify these rare conditions. They [could] support our providers in trying to diagnose these patients and get them the care that they need.”
Considerations in Medical/Pharmacy Benefit Design for Rare Disease Therapy
As discussed earlier, sometimes it is beneficial for providers to bill through the medical benefit so patients have coverage for treatment and it can be given in the most appropriate setting. However, payers in attendance at the roundtable meeting indicated that the pharmacy benefit is seen by some as more consistent and that with it, it’s easier to control costs than with the medical benefit. “From a managed care standpoint, the pharmacy benefit does allow a greater sense of consistency in costs, especially in rare disease management, as you start getting into specialists and centers of excellence [COEs] and their costs,” Spence shared. “As a plan, on the medical benefit, we have a higher risk of variable unit cost. Leveraging the pharmacy benefit helps to control some of that.” Data integration across the medical and pharmacy benefit would help payers assess the total cost of care of using a drug in the real world and subsequently guide treatment decisions.
Payer and Provider Support for Patients With Rare Diseases
A national disease registry would help payers and physicians understand and provide the needed support for patients with rare diseases, emphasized Stephens. “A national disease registry [that included] everyone with that disease across payer mix plans, states, locations, geographic [areas], [and] demographics” would be very useful, he said. With that, “you could start to accumulate the data that you need to look at incidence, prevalence, treatments, options, outcomes.”
CARE MANAGER AND CASE MANAGER SUPPORT FOR PATIENTS WITH RARE DISEASES
As Cannon pointed out, in the rare disease category, there isn’t always a formulary decision to be made. For some rare or ultrarare diseases, only 1 treatment option may exist. Payers will not deny a person such therapy, according to Cannon; they will just ensure that patients are receiving the correct medication for their diagnosis. Care managers, who help patients sort through the health care system once a diagnosis is complete, are critical to improved health care delivery, “because patients just don’t know where to go, and they just don’t know how to get in to see the appropriate physicians,” stated Cannon.
Other participants echoed those sentiments, especially concerning patients with rare diseases. “It’s very challenging for the average patient who’s not health care–literate and possibly not even extremely literate to begin with,” Davis expressed, “to be thrust into this space, where they have so many challenges and things to understand. The simple things that a case manager or care coordinator can do for you changes the life of the family. I would just say they’re a critical part of the health care team.”
Care coordination and management can also benefit infants or children with rare diseases and their parents. “Sometimes [case managers] walk alongside these patients. They are the voice of the patients, because they’re so young,” said Williams. “[Sometimes] the parents are working, tired, or [overwhelmed just by] taking the child back and forth to the doctor’s office, so they help [the parents] navigate the payer system.” Regarding other patients, noted Williams, “if you [have previously been mostly] healthy, and you’ve only had to use your insurance for certain things, you don’t know how to use your insurance and who to call. I think patient navigators, patient advocates, are huge connectors here. The more we have, [the more] we can really help some of these patients who don’t have voices. [Also, we need] a national electronical medical record (EMR) that integrates the overall information, because there are times that a patient goes to the hospital, and their own doctors don’t know. They’re not informed until later on. I think both of them [patient navigators and EMR] can play a part in helping these patients.”McCormick added that advocates have resources that they can bring in if patients need a nurse, a pharmacist, or behavioral health support. “We have gone more to that model of … managing overall health.” Care managers play a valuable role that is “constantly evolving,” agreed Stephens. In children with rare diseases, such as HLH, he said, it is critical to “keep the parent on a hotline, [to make sure they are] informed, [have] information, so … they … know when to go to the doctor, when to call the [ED] and do prophylactic visits through providers… [even] … on holidays and weekends.”
Advocates can also play a valuable role in relieving the burdens of caregivers, especially those caring for patients with rare, often unpredictable, disease courses. For example, caregivers of patients with hATTR report high levels of fatigue.4 In addition, both patients with hATTR and their caregivers face significant mental health burdens. Care managers can help patients and caregivers find appropriate support.
Pharmaceutical companies are also expanding patient education and support services. For example, Akcea Therapeutics (now an Ionis company) established the Rare Disease Therapeutic Resource Center with Accredo specialty pharmacy to provide patient education and outreach services to improve health outcomes, reduce time to receipt of therapy, and lower overall health care costs. In conjunction with Accredo’s team of specialty clinicians, pharmacists, and more than 600 field-based nurses located throughout the United States, Akcea assembled a special team of nurse case managers to provide support to patients with hATTR and focus on their needs.20
LOOKING FORWARD: OPPORTUNITIES TO IMPROVE PATIENT CARE IN RARE DISEASE
Misdiagnosis of rare diseases, such as hATTR and HLH, can occur frequently, because symptoms of the rare disease may present as nonspecific. Nonetheless, early diagnosis is key to ensure that patients receive optimal care as soon as possible. Payers’ roles expand once a diagnosis has been made. Therefore, education to providers about rare diseases is essential to improve diagnoses and outcomes. Khella indicated that pharmaceutical companies have made a concerted effort to educate neurologists about hATTR, for example, helping them recognize when this condition should be on their radar in patients with neuropathy.
When considering utilization strategies, payers approach acute and chronic diseases differently, said Spence. She indicated that duration of therapy must be considered, which can also influence cost. There is a growing interest in policies for chronic therapies outside of the core offering at a health plan, such as stop-loss or other types of financial risk management strategies. The coverage approach is more data-driven for acute therapies, and it involves policies with competitive contract rates for providers and participation from 340B-covered entities to help mitigate the cost for those 1-time claims.
It is important for payers and providers to support patients with rare diseases and their caregivers. Payers and physicians can work together to improve patient care by looking at all components that go into effective disease management—everything from prescriptions to transportation for follow-up visits. The Institute of Medicine recognizes care coordination (ie, care management) as a primary strategy to help enhance effectiveness, safety, and efficiency in the health care system. Coordinated care is the deliberate organization of patient care activities and information sharing across all care providers to achieve safer, more effective care.21,22
Pharmaceutical manufacturers and payers may partner together to improve outcomes in rare disease. Roundtable participants indicated that there may be an opportunity for value-based agreements (VBAs), where coverage and reimbursement are linked to the effectiveness and utilization frequency of a drug. VBAs may be useful for rare disease products, because they help manufacturers separate their product from those of competitors based on agreed-upon outcomes, and they can inform payers about the clinical value, efficacy, and financial implications of a new drug.18
Registries offer a useful way to collect real-world data in rare disease. The Transthyretin Amyloidosis Outcomes Survey is a global, longitudinal, observational survey open to all patients with TTR amyloidosis. The registry aims to better understand and characterize the natural history of the disease. Data can be used to develop new treatment guidelines and management recommendations.23 Other registries for rare diseases are available from Orphanet (European countries)24; the National Center for Advancing Translational Sciences’ Rare Diseases Registry Program25; and the IAMRARE Registry Program from the National Organization for Rare Disorders (NORD).26
Patients with rare diseases may need care that presents a financial strain. Current plans are providing specialized care for rare disease through identification of “high-cost claimants” and the suggestion of nurse navigators to help the patient/caregiver. Some rare disease organizations provide financial information and assistance programs to help patients manage costs. For example, NORD’s RareCare program offers medication, financial assistance with insurance premiums and co-pays, diagnostic testing help, and travel reimbursement to patients with a rare disease.27
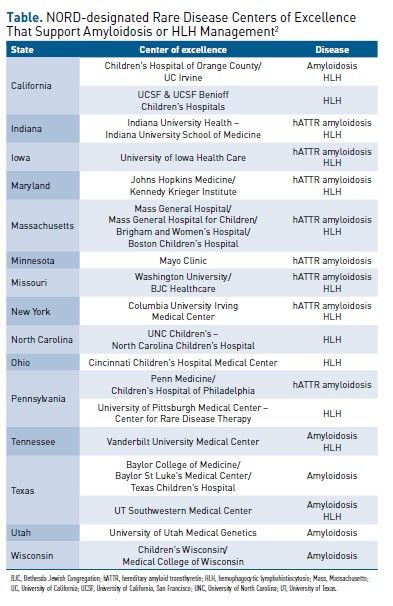
COEs offer specialized care for rare diseases affecting adults and children; such care is critical to ensure the best outcomes. Recently, NORD designated 31 medical centers, clinics, and institutions across the United States as COEs in rare disease.2 These COEs are located in 21 states and Washington, DC, and they manage numerous rare diseases. COEs with management expertise in amyloidosis or HLH are listed in the Table.2
CONCLUSIONS
This discussion of experts, both provider and payer, explored utilization management tactics, drug benefit design, and implications of site of care on patient access within the rare disease space. hATTR and HLH are 2 areas in chronic and acute rare diseases with unmet needs where improved access to care is needed. Education to providers about rare diseases is essential to help prevent misdiagnoses and improve outcomes.
REFERENCES
1. Rare diseases. National Institutes of Health. Updated February 11, 2020. Accessed December 15, 2021. https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/rare-diseases
2. Committing to care, access, equity and research: NORD announces 31 Rare Disease Centers of Excellence. News release. National Organization for Rare Disorders. November 4, 2021. Accessed December 15, 2021. https://rarediseases.org/committing-to-care-access-equity-and-research-nord-announces-31-rare-disease-centers-of-excellence/
3. Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625-638. doi:10.3109/07853890.2015.1068949
4. Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;23(suppl 7):S107-S112.
5. Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31. doi:10.1186/1750-1172-8-31
6. Ueda M, Ando Y. Recent advances in transthyretin amyloidosis therapy. Transl Neurodegener. 2014;3:19. doi:10.1186/2047-9158-3-19
7. Arno S, Cowger J. The genetics of cardiac amyloidosis. Heart Fail Rev. Published online September 13, 2021. doi:10.1007/s10741-021-10164-z
8. Ruberg FL, Maurer MS, Judge DP, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;164(2):222-228.e1. doi:10.1016/j.ahj.2012.04.015
9. Onpattro. Prescribing information. Alnylam Pharmaceuticals; 2021. Accessed November 18, 2021. https://www.alnylam.com/wp-content/uploads/pdfs/ONPATTRO-Prescribing-Information.pdf
10. Tegsedi. Prescribing information. Akcea Therapeutics; 2021. Accessed November 18, 2021. https://tegsedihcp.com/pdf/prescribing-information.pdf
11. Vyndaqel and Vyndamax. Prescribing information. Pfizer; 2019. Accessed November 18, 2021. https://www.fda.gov/media/126283/download
12. Value-based agreements in rare disease: focus on hereditary amyloid transthyretin amyloidosis. American Journal of Managed Care® Supplements and Featured Publications. January 27, 2021. Accessed December 21, 2021. https://www.ajmc.com/view/value-based-agreements-in-rare-disease-focus-on-hereditary-amyloid-transthyretin-amyloidosis
13. Henter JI, Elinder G, Söder O, Öst A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80(4):428-435. doi:10.1111/j.1651-2227.1991.tb11878.x
14. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
15. La Rosée P, Horne AC, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
16. Gamifant. Prescribing information. Sobi; 2018. Accessed November 18, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761107lbl.pdf
17. Hilgers RD, König F, Molenberghs G, Senn S. Design and analysis of clinical trials for small rare disease populations. J Rare Dis Res & Treat. 2016;1(3):53-60. doi:10.29245/2572-9411/2016/3.1054
18. Novel advances in hereditary amyloid transthyretin amyloidosis: a stakeholder interchange report. American Journal of Managed Care® Supplements and Featured Publications. February 7, 2020. Accessed December 21, 2021. https://www.ajmc.com/view/novel-advances-in-hereditary-amyloid-transthyretin-amyloidosis-a-stakeholder-interchange-report
19. Kittleson MM, Maurer MS, Ambardekar AV, et al; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1):e7-e22. doi:10.1161/CIR.0000000000000792 Published corrections appear in Circulation. 2021;144(1):e10 and Circulation. 2021;144(1):e11.
20. Akcea announces its access and distribution strategy for Tegsedi (inotersen). News release. Ionis Pharmaceuticals. October 5, 2018. Accessed December 15, 2021. https://ir.ionispharma.com/news-releases/news-release-details/akcea-announces-its-access-and-distribution-strategy-tegseditm
21. Care coordination. Agency for Healthcare Research and Quality. Reviewed August 2018. Accessed December 15, 2021. https://www.ahrq.gov/ncepcr/care/coordination.html
22. Gionfriddo MR, Pulk RA, Sahni DR, et al. ProvenCare-Psoriasis: a disease management model to optimize care. Dermatol Online J. 2018;24(3):13030/qt5xt2s05b.
23. Transthyretin Amyloidosis Outcome Survey (THAOS). ClinicalTrials.gov. Updated November 9, 2021. Accessed December 15, 2021. https://clinicaltrials.gov/ct2/show/NCT00628745
24. Research and trials/registries & biobanks. Orphanet. Accessed December 15, 2021. https://www.orpha.net/consor/cgi-bin/ResearchTrials_RegistriesMaterials.php?lng=EN
25. Rare Diseases Registry Program (RaDaR). National Center for Advancing Translational Studies/National Institutes of Health. Updated April 15, 2021. Accessed December 15, 2021. https://ncats.nih.gov/radar
26. IAMRARE Registry Program. NORD: National Organization for Rare Disorders. 2021. Accessed December 15, 2021. https://rarediseases.org/iamrare-registry-program/
27. RareCare. NORD: National Organization for Rare Disorders. 2021. Accessed December 15, 2021. https://rarediseases.org/for-patients-and-families/help-access-medications/patient-assistance-programs-2/#section-1

