- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Pharmacogenomics for Improved Outcomes and Decreased Costs in Health Care
This article was sponsored by Payer Matrix.
Pharmacogenomics
Numerous physiologic, pathophysiologic, and lifestyle factors influence a drug’s pharmacokinetic (PK) and pharmacodynamic (PD) profile in individuals; genetic contributions in particular play a key role.1 Patients’ responses to medications vary within therapeutic categories.2 Although most drugs have a 50% to 75% response rate, some medications (eg, those used in oncology) may elicit a response in as little as 25% of patients.2
Pharmacogenomics (PGx) involves the study of patients’ unique genes to predict their individual response to drugs.1,3 In assessments of the 12 most important pharmacogenes, at least 1 clinically actionable pharmacogenetic variant has been found in at least 90% of people.4-6 Thus, information culled from PGx diagnostics can provide important information about safety and efficacy associated with various pharmaceuticals with different dosage profiles in a tested patient.
The results of a 6-year cross-sectional study of more than 7.7 million US veterans showed that approximately 55% had a medical record for a level-A agent (ie, medications with a strong recommendation to avoid or adjust the dose based on PGx results).7 Outcomes also showed that nearly 38% received a prescription for a new level-A drug; nearly 25% of these patients were prescribed 2 level-A agents, and approximately 12% were prescribed 3 or more level-A medications. Receipt of multiple level-A prescriptions may further increase the risk of a poor or unfavorable response.7
Genetic variations can affect drug efficacy and safety.Differences in genes affecting pharmacokinetic parameters (eg, drug absorption, distribution, metabolism, and elimination) impact systemic exposure and indirectly influence response to a drug. Similarly, variations in genes that affect a drug’s pharmacodynamics, notably its therapeutic on- and off-target sites, directly influence response.8
Historically, medications were prescribed to patients based on a standardized, empirical approach that involved adjusting for nonoptimized medication therapy (ie, therapy failure or a clinical symptom or syndrome caused by a new therapy) through trial and error.8,9 Now, in the age of precision medicine and individualized patient care, PGx can help to identify genetic predictors for patient response to guide the selection of an appropriate therapy. PGx testing can increase the likelihood of patients being prescribed the correct medication at the point of care, and it may enable more prompt administration of the correct dosage (Figure).4,8,10 PGx-driven medication therapy management programs and other tools can improve medication adherence, ensure medication appropriateness, and reduce adverse drug reactions (ADRs) early in the course of treatment. Selection of medications that may be associated with fewer ADRs may generate better outcomes.11,12
Figure. Pharmacogenetics Testing for Early Administration of the Correct Medication at the Correct Dose4,8-10
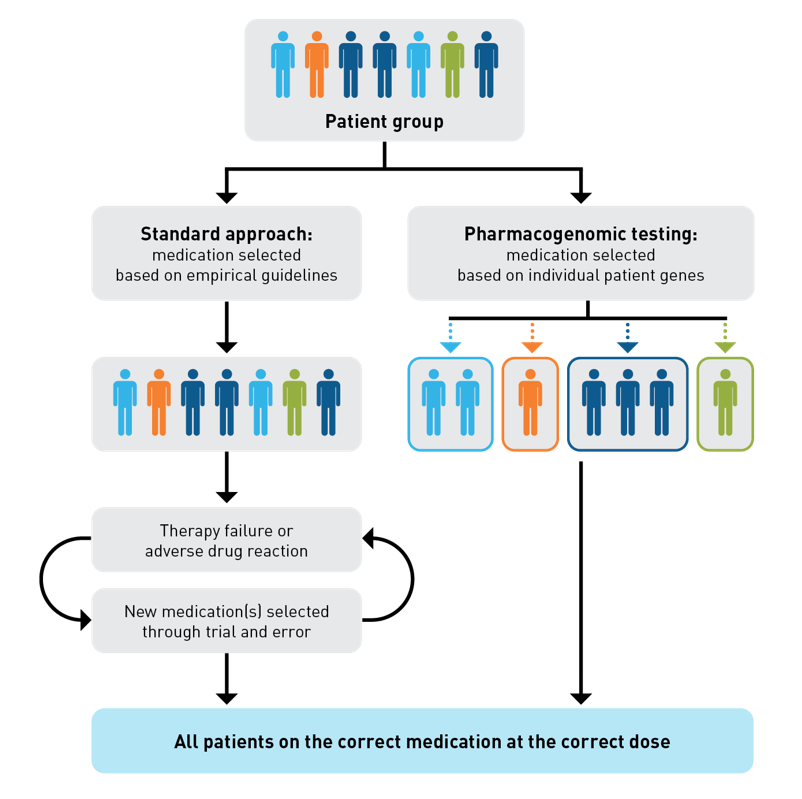
Agencies, like Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG), are continuously developing guidelines for PGx testing, andthe information for more than one hundred drug-gene pairs are already published.13-15 There is a plethora of evidence demonstrating that the administration of medications supported by PGx test results in has led to decreased hospitalizations, emergency department visits, inpatient days, and health care costs.4,16,17 These literature case studies very emphatically suggest that PGx testing will become a dynamic strategy with a strong potential to generate considerable cost-savings, and improve outcomes for employers, payers, providers, and patients. The implementation of PGx testing and diagnostics across entire health care systems can have far-reaching effects, including enhancement of operational efficiency and optimization of patient care.17
High Cost of Treatment Failure and Adverse Drug Events (ADRs)
According to the FDA (Food and Drug Administration), ADRs are one of the greatest contributors to morbidity and mortality in health care.18 Overall, according to results from a 2018 analysis using national data sources to model cost probabilities of prescription medication-related morbidity and mortality, in 2016 nonoptimized prescription medication therapy was associated with approximately 275,689 deaths and linked to some 16% of total health care expenditures ($3.2 trillion in 2015) in the United States.9,19 The estimated annual direct medical costs associated with nonoptimized medication therapy were $528.4 billion (range, $495.3 billion to $672.7 billion).9 As published, accounting for the use of additional medical resources such as hospitalizations and provider visits, this cost was considered potentially avoidable.9 Although genetics may not account for all cases involving nonoptimized medication therapy, drug prescriptions guided by PGx testing can potentially reduce clinically relevant ADRs by 30%.4 Based on the previously described 2016 estimated annual cost of $528.4 billion for nonoptimized medication therapy, an interpretation may be that use of this technology could translate into annual savings of several billion dollars across the US health care industry.9
PGx Testing
PGx testing informs prescribers with results that can improve drug safety (eg, findings on Human Leukocyte Antigen: HLA-B*57:01 that may affect abacavir use) or impact efficacy (eg, information about Cytochrome 450: CYP2C19 may impact clopidogrel therapy; data on CYP2D6 and CYP2C19 may affect use of selective serotonin reuptake inhibitors).20-22 Although PGx testing is often considered for a particular gene or set of genes and a specific drug or therapeutic class of drugs, analysis using multigene PGx panels can serve as an important prescribing tool.23 Information on 1 or 2 genes can affect numerous drugs representing different drug classes.23 An example is CYP2C19 genotyping, which can provide information relevant to therapeutic decisions for antidepressants, clopidogrel, proton pump inhibitors, and voriconazole. Although PGx testing results may initially apply to 1 medication, multigene panel results may be useful for patients who require multiple medications later in life.23
Improved Outcomes and Cost Savings
Results from a recent retrospective study by Jarvis et al demonstrated that, when combined with comprehensive medication management (CMM) services, PGx testing can improve appropriate drug selection, support drug adherence, and reduce ADRs—which ultimately resulted in better patient outcomes and considerable cost savings.16 The services included patient education, genetic testing, pharmacist review, and a comprehensive medication action plan and were offered to Medicare-eligible health plan members in the Teachers’ Retirement System Kentucky.
Overall, participants who received the services (the intervention group; n = 5288) experienced fewer inpatient days, fewer emergency department visits, and more outpatient visits than did the control group (n = 22,357). Direct medical charges decreased in the intervention group by approximately $7000 per patient overall, or an average of $218.82 per patient per month. During the study’s 32 months, cost savings totaled $37 million.16 Additionally, the program combining PGx with CMM shifted health care resource use to sustainable and cost-effective primary care when compared with more resource-intensive acute care. The authors concluded that this approach that combined PGx and CMM could support achievement of each goal of the quadruple aim: improvement in outcomes, costs, patient experience, and clinician experience.16
The benefit derived from CMM combined with PGx services was also demonstrated in a case report of a patient (female; age 71 years) who had also participated in a CMM plus PGx program through her retirement benefit. At her medication review, the patient reported a treatment regimen of 9 medications and supplements, including once daily metoprolol succinate 25 mg and simvastatin 40 mg. Symptoms she experienced were fatigue, periodic dizziness that occurred when standing, nocturnal leg pain or cramping, and low energy; she attributed these symptoms to her compromised cardiovascular health and older age and therefore had not reported them to her physician.24 The PGx test showed atypical results for genes CYP2D6 and SLCO1B1, which indicated inefficiency in metoprolol metabolism and simvastatin uptake in the liver.24 Based on these insights, the pharmacist had recommended switching 25 mg metoprolol succinate to 5 mg bisoprolol and switching 40 mg simvastatin to 5 mg rosuvastatin. The patient initiated this new regimen. At 18 months following medication review, her blood pressure and cholesterol levels remained moderately controlled, and she reported improvements in leg pain, sleep, energy, and overall quality of life. The authors of the case report stated that the sustained (more than 18 months) reduction in symptoms suggested the symptoms were due to medication side effects, and that PGx testing played an important role in optimizing treatment tolerability for this patient.24
As mentioned earlier, PGx testing can reduce ADR risk by up to 30%.4 These results were demonstrated in a prospective study conducted across 7 European countries that explored the use of a 12-gene pharmacogenomic panel in prescribing medications.4 Participants had received a prescription medication that was included in the DPWG guidelines. Outcomes from a first gatekeeping analysis showed that, of patients who had an actional variant for their medication (n = 1558), 21% (152/725) in the PGx intervention group and 28% (231/833) in the control group experienced clinically relevant ADRs, which demonstrated a 30% reduced risk of ADRs for those who received PGx testing (odds ratio .70 [95% CI, 0.54–0.91]; P = .0075). A second gatekeeping analysis, which included patients both with and without an actionable variant (n = 6193), produced similar results.4 The authors noted that their outcomes were in line with the results from Jarvis et al, both of which supported the use of PGx panel testing.4
Overall Cost Effectiveness
Implementation cost often is considered to be a primary barrier to PGx use among health care systems, and payers require insight into PGx cost-effectiveness when determining coverage benefits.23,25 However, in 2017 the cost of PGx tests had been decreasing since 2009 and test availability had been increasing; therefore, it was projected that eventually genotype information could be a part of every electronic health record at a negligible price.26 A comprehensive literature review of publications through June 2021 assessed 108 studies regarding the cost-effectiveness of PGx-guided treatments for drugs included in CPIC guidelines.23 Of the studies reviewed, the results of 71% found that use of PGx testing was cost-effective or cost-saving, of 20% determined that it was not cost-effective, and of 9% noted uncertainty that testing was beneficial.23 These outcomes show that the 1-time, fixed costs of PGx implementation and testing can be eventually offset by the downstream savings.23
The offset of initial test costs was demonstrated in a study that assessed cost and outcome data associated with depression to model potential savings (adjusted to 2016 dollars) with use of PGx. Results showed that, accounting for the upfront $2,000 cost of each PGx test used per patient, ultimate downstream savings were $3,962 per patient per year.27 Of note, the upfront cost of PGx tests can be considerably lower than that used in the study, with bills for single-gene tests ranging as low as approximately $200 to $400 and multi-gene tests up to double the cost of single-gene tests.28,29
Conclusions
PGx testing has a strong potential as a tool for health systems and payers, as it individualizes patient care to improve outcomes and reduce associated cost burdens.4,8,16 With clinical guidelines established for more than 100 drug-gene pairs, providers can use PGx testing to select drugs with an optimal efficacy and safety profile for each patient, thus improving the likelihood of an effective response and decreasing the risk of ADRs and associated morbidity and death.11,13,18 With a demonstrated ability to reduce inpatient days and emergency department visits and considerably decrease direct medical costs in tested populations, the use of PGx with CMM can support providers in achieving the quadruple aim.16 Future directions for genetic testing in personalized medicine include identification of additional gene markers and associated medications and assessment of the impact of nutrition on genetic expression (nutrigenomics).These tools offer even more promise in optimizing health care delivery, and their use may further improve the efficacy and safety of necessary medications.30,31
Payer Matrix is a heath care cost containment company based in Pennsylvania, focused on providing quality care management and advocacy for specialty drugs, and providing a substantial cost saving to clients and members.
References
- Topic E. 5. Pharmacogenomics and personalized medicine. EJIFCC. 2008;19(1):31-41.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7(5):201-204. doi:10.1016/s1471-4914(01)01986-4
- Evans WE, McLeod HL. Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538-549. doi:10.1056/NEJMra020526
- Swen JJ, van der Wouden CH, Manson LE, et al; Ubiquitous Pharmacogenomics Consortium. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401(10374):347-356. doi:10.1016/S0140-6736(22)01841-4
- Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol 2015;55:89-106. doi:10.1146/annurev-pharmtox-010814-124835
- Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95(4):423-431. doi:10.1038/clpt.2013.229
- Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
- Rollinson V, Turner R, Pirmohamed M. Pharmacogenomics for primary care: an overview. Genes (Basel). 2020;11(11):1337. doi:10.3390/genes11111337
- Watanabe JH, McInnis T, Hirsch JD. Cost of prescription drug-related morbidity and mortality. Ann Pharmacother. 2018;52(9):829-837. doi:10.1177/1060028018765159
- Pirmohamed M, Burnside G, Eriksson N, et al; EU-PACT Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294-2303. doi:10.1056/NEJMoa1311386
- 1Dorfman R, London Z, Metias M, Kabakchiev B, Mukerjee G, Moser A. Individualized medication management in Ontario long-term care clinical impact on management of depression, pain, and dementia. J Am Med Dir Assoc. 2020;21(6):823-829.e5. doi:10.1016/j.jamda.2020.04.009
- Berndt ER, Goldman DP, Rowe J. The value of pharmacogenomic information. In: Economic Dimensions of Personalized and Precision Medicine. The University of Chicago Press; 2019:53-86.
- Abdullah-Koolmees H, van Keulen AM, Nijenhuis M, Deneer VHM. Pharmacogenetics guidelines: overview and comparison of the DPWG, CPIC, CPNDS, and RNPGx guidelines. Front Pharmacol. 2020;11:595219. doi:10.3389/fphar.2020.595219
- Guidelines. CPIC website. Accessed October 30, 2023. https://cpicpgx.org/guidelines/
- Home page. PharmGKB website. 2023. Accessed October 30, 2023. https://www.pharmgkb.org/
- Jarvis JP, Peter AP, Keogh M, et al. Real-world impact of a pharmacogenomics-enriched comprehensive medication management program. J Pers Med. 2022;12(3):421. doi:10.3390/jpm12030421
- Ghandi M. Improve lives, reduce costs and simplify decision-making: enterprise pharmacogenomics. ThermoFisher Scientific. February 2, 2022. Accessed October 30, 2023. https://www.thermofisher.com/blog/clinical-conversations/improve-lives-reduce-costs-simplify-decision-making-enterprise-pharmacogenomics/
- Preventable adverse drug reactions: a focus on drug interactions. FDA. March 6, 2018. Accessed November 8, 2023. https://www.fda.gov/drugs/drug-interactions-labeling/preventable-adverse-drug-reactions-focus-drug-interactions
- Historical. CMS website. Accessed November 13, 2023. https://www.cms.gov/data-research/statistics-trends-and-reports/national-health-expenditure-data/historical
- Martin MA, Klein TE, Dong BJ, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Therapeut. 2012;91(4):734-738. doi:10.1038/clpt.2011.355
- Scott SA, Sangkuhl K, Stein CM, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317-323. doi:10.1038/clpt.2013.105
- Hicks JK, Bishop JR, Sangkuhl K, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Therapeut. 2015;98(2):127-134. doi:10.1002/cpt.147
- Morris SA, Alsaidi AT, Verbyla A, et al. Cost effectiveness of pharmacogenetic testing for drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines: a systematic review. Clin Pharmacol Ther. 2022;112(6):1318-1328. doi:10.1002/cpt.2754
- Prather A, Aifaoui A, Shaman JA. Idiopathic symptoms resolved by pharmacogenomics-enriched comprehensive medication management: A case report. Cureus. 2022;14(2):e21834. doi:10.7759/cureus.21834
- Jameson A, Fylan B, Bristow GC, et al. What are the barriers and enablers to the implementation of pharmacogenetic testing in mental health care settings? Front Genet. 2021;12:740216. doi:10.3389/fgene.2021.740216
- Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017;17(5):395-402. doi:10.1038/tpj.2017.21
- Maciel A, Cullors A, Lukowiak AA, Garces J. Estimating cost savings of pharmacogenetic testing for depression in real-world clinical settings. Neuropsychiatr Dis Treat. 2018;14:225-230. doi:10.2147/NDT.S145046
- 2Haidar CE, Crews KR, Hoffman JM, Relling MV, Caudle KE. Advancing pharmacogenomics from single-gene to preemptive testing. Annu Rev Genomics Hum Genet. 2022;23:449-473. doi:10.1146/annurev-genom-111621-102737
- Anderson HD, Crooks KR, Kao DP, Aquilante CL. The landscape of pharmacogenetic testing in a US managed care population. Genet Med. 2020;22(7):1247-1253. doi:10.1038/s41436-020-0788-3
- Singh V. Current challenges and future implications of exploiting the omics data into nutrigenetics and nutrigenomics for personalized diagnosis and nutrition-based care. Nutrition. 2023;110:112002. doi:10.1016/j.nut.2023.11200231.
- Pirmohamed M. Pharmacogenomics: current status and future perspectives. Nat Rev Genet. 2023; 24:350-362. https: 10.1038/s41576-022-00572-8
