- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Payer and Provider Solutions to Utilization Management Challenges in the Management of Rare Hematologic Cancers
ABSTRACT
Patients with rare diseases such as Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), a hematologic malignancy affecting approximately 1500 new patients per year, experience barriers to care involving both clinical and administrative factors. Optimal patient outcomes depend on timely identification, diagnosis of disease, and treatment initiation. For patients living with Ph+ ALL, the process can be delayed by limited treatment options approved by the US Food and Drug Administration and administrative hurdles that often delay treatment initiation. An overhaul of utilization management processes, such as the requirement for prior authorization (PA) for treatment, are needed to ensure patients have access to appropriate treatments in a timely manner. An AJMC Roundtable in November 2022 brought together a panel of payers and providers to discuss the challenges and shortcomings of current PA processes and to present ideas for potential solutions for improving them. Panelists at the roundtable discussed approaches including the use of guideline-concordant electronic PAs and other digital solutions, expedited approval pathways for use in specific conditions, use of real-world evidence in decision-making, issuance of PA “Gold Cards” to select providers, and a shift to value-based care agreements. Roundtable attendees agreed that, regardless of the strategy for PA-process improvement, there is a need for improved communication between providers and payers to ensure that the decision-making system meets the essential need for timely patient access to optimal care. This article reviews utilization management and guideline-concordant care through the lens of rare diseases and then presents solutions to utilization management challenges to expedite access to therapy.
Am J Manag Care. 2023;29(suppl 4):S51-S60. https://doi.org/10.37765/ajmc.2023.89365
For author information and disclosures, see end of text.

Introduction
Rare cancers are defined by the National Cancer Institute as any cancer type that affects fewer than 15 of every 100,000 people each year.1 Accordingly, in a 1 million–member health plan, very few patients will be affected by rare cancers of any type.
Patients with rare hematologic malignancies often have specific management needs requiring the expertise of specialized centers and practitioners. For example, challenges in timely and accurate diagnosis and characterization of acute lymphoblastic leukemia (ALL) often leads to poor outcomes. Additionally, implementation of peer-reviewed guidelines and protocols for rare cancers such as ALL can be challenging for community-based hematologists or oncologists who see these conditions infrequently.2
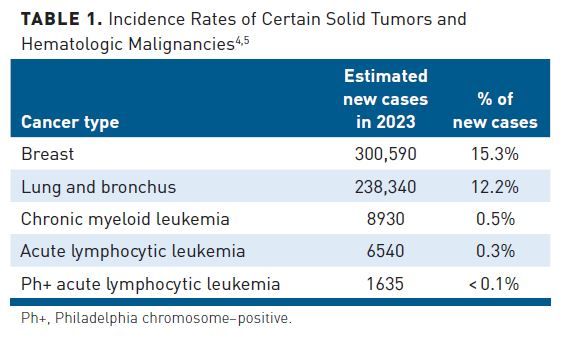
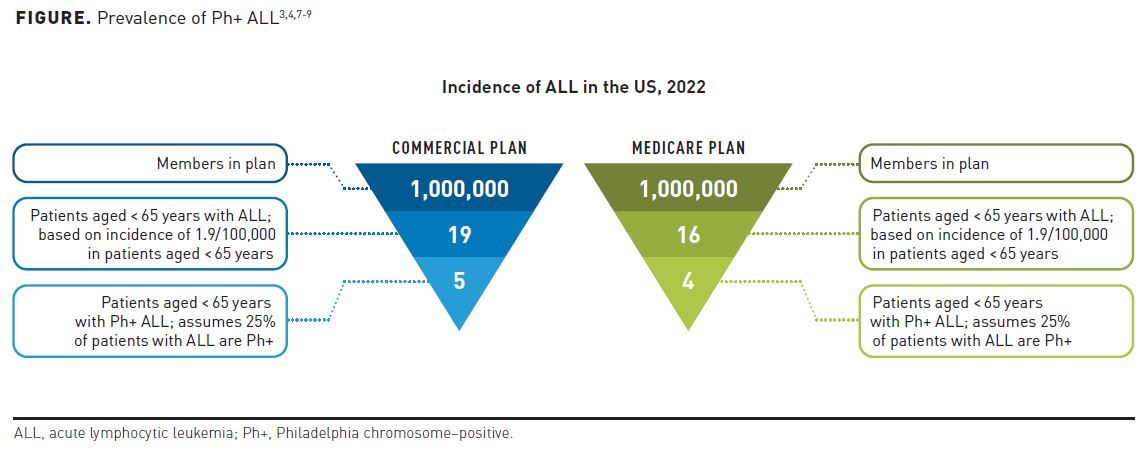
In ALL, a hematologic cancer affecting more than 6000 new patients per year, the age-adjusted incidence rate is 1.8 per 100,000 men and women per year (Table 1).3-5 Philadelphia chromosome-positive (Ph+) ALL is characterized by the BCR:ABL fusion, found in approximately 25% of patients with ALL and representing the most common cytogenetic abnormality in adult ALL.5,6 In a 1 million–member health plan, payers would expect to see approximately 4.5 new cases of Ph+ ALL per year (Figure).3,4,6-9
As with other rare cancers, timely detection of ALL is critical for effective and timely treatment, which may lead to better survival outcomes.10 Outcomes for patients treated with ALL have improved over past decades, largely due to enhanced understanding of the pathogenesis of disease and the refinement of treatment algorithms and guidelines for care. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®), which are published and updated multiple times per year, offer treatment information for patients and health care providers and are designed to improve patient outcomes through evidence-based assessment of optimal treatment patterns, safety, and efficacy.11
Considerations and Unmet Needs in Rare Hematologic Cancers: Acuity of Care
Despite improvements in outcomes over the past decade, several challenges remain regarding patient care for those diagnosed with rare hematologic cancers.12-13 Delayed identification, diagnosis, and treatment initiation may lead to disease progression, higher morbidity rates, and higher costs associated with health care resource utilization.
Despite the development of treatment options for patients with solid tumors, treatment options for leukemia are limited and may have more barriers to access. Specifically in rare hematologic cancers, such as Ph+ ALL, an already finite set of treatment options may be limited by the site of care. For a cancer with poor prognosis, timeliness to treatment is critical and may be further affected by factors such as access to transportation and other social determinants of health. Although it is imperative for a patient to start on treatment quickly, it is equally important to ensure the patient receives the right treatment.
Provider roundtable participants Jabbour and Geyer support the idea that there are characteristics of rare hematologic cancers, such as Ph+ ALL, that necessitate expedited treatment approval time from payers. (This will be explored as a case study later in this article.) Unlike in several common solid tumors for which multiple frontline treatment options may be appropriate, in certain rare hematologic malignancies, fewer appropriate treatment options may be available. In patients with Ph+ ALL, for example, the backbone of treatment is one of a handful of potent ABL-targeted tyrosine kinase inhibitors (TKIs). Less rare types of cancer (eg, breast, prostate, lung) may be most cost-effective to manage in the outpatient setting; however, there are several considerations to support a different approach for the management of rare hematologic cancers with acute needs that benefit from immediate treatment in the inpatient setting.
Utilization management (eg, step therapy and prior authorizations [PAs]) in rare cancers, specifically rare hematologic cancers, can present a challenge for providers to initiate treatment. This is an area of opportunity to improve disease management and understanding among payers that only limited treatment options can be used and the treatment setting may impact the time to start of care.
In addition to timeliness of diagnosis and treatment initiation, access to treatment is another barrier. In some settings, access to treatments can be delayed by the need for payer approval. Once this approval is obtained, many of these treatments are not readily available at the care site and must be obtained from a specialty pharmacy. These administrative and logistical delays to treatment initiation may further complicate patient care and outcomes.
Jabbour explained that at The University of Texas MD Anderson Cancer Center, which is a hospital/inpatient acute care setting, payer approval is sought before treatment initiation. The hospital has an inpatient formulary, but when discharging a patient with commercial coverage, payers often want to ensure that the therapy is covered on the pharmacy benefit or preferred specialty pharmacy’s formulary. Geyer expanded that another part of this issue is not whether the drug is on formulary but if it is available in the hospital. “If [a patient] is in the ICU in the hospital, [payer] approval is needed for the drug to be sent to specialty pharmacy,” he explained. “I cannot just dispense it. I don’t have it on the shelf.” In this setting, providers rely on patients to secure their own supply of medications outside of inpatient care.
With an evolving treatment landscape, it may be difficult for providers to stay up to date with the most recent advancements in rare disease treatment, potentially leading to suboptimal treatment regimens and a diminished standard of care. Better cancer management, individually tailored towards each patient’s needs and goals, may enhance quality-of-care outcomes while also lowering overall cost of care.
Impact of PA on Timely and Appropriate Care
Utilization management, formerly utilization review, refers to an evaluation of the clinical suitability and necessity of treatments, services, or procedures to manage costs and reduce waste.14,15 Utilization management interventions can occur prior to a clinical event (a PA) while a patient is in a health care facility (a concurrent review) or after a clinical encounter (a retrospective review).14 However, these utilization management hurdles may sometimes lead to delays in treatment initiation, which may prevent patients with rare diseases from receiving timely care.
Utilization Management Supports Appropriate Patient Care
The purpose of PA is to ensure that the physician-requested treatment, service, or procedure is medically appropriate and will be delivered in the appropriate setting. In addition to helping manage costs, a PA should facilitate a conversation about a patient’s care plan. This conversation may encourage more effective coordination of patient care.14 PAs also may be used to support ongoing patient treatment, specifically for medications with a potential for misuse or with long-term safety concerns.15
From the payer perspective, PAs offer another level of support to review and confirm that a patient’s plan of care is the best medical option and to ensure that the treatment is covered by the plan. During the AJMC Roundtable, payer participants emphasized the potential value of PAs to improve care for underrepresented patients. For example, those living in rural areas may not have access to care through specialists or experts in the field, such as those in large institutions like Memorial Sloan Kettering Cancer Center or MD Anderson. In those cases, PAs can help by facilitating access to treatments or ensuring access to the appropriate therapies. Payer participants viewed the role of PAs as that of helping to ensure their members get the best possible care in alignment with guideline-concordant care. One participant agreed that payers see their role as helping to ensure that members get the best quality care possible by providing a second set of eyes to review the recommended treatment. Payers recognize that they have a responsibility both to providers and to members in terms of ensuring that patients get the right care at the right time.
The PA Process as a Barrier to Patient and Provider Access
Although some may see the PA process as an important step in getting patients proper care, provider experience suggests that PA requirements may create administrative barriers to accessing a necessary medication in a timely manner, which can have clinical implications for patients and treatment outcomes.
A 2022 American Medical Association (AMA) Physician Survey documented the substantial administrative burden associated with obtaining a PA. Submitting a PA is a manual, time-consuming process that can burden providers and divert valuable resources away from direct patient care. Medical practices surveyed in the AMA report completed an average of 45 PAs per physician per week, which translated to an average of 14 hours of work per week. The burden of submitting PAs was reported as “high” or “extremely high” by 88% of physicians, and 35% of practices reported having full-time staff that work exclusively on submitting PAs.16
Geyer cited this burden as substantial enough at his institution to require the implementation of a separate workflow process to handle the PA volume and to necessitate the hiring of additional team members in the Patient Financial Services department to alleviate the burden and help manage the PA process.
Results of the 2022 AMA survey also showed that, in addition to administrative concerns, 89% of physicians reported that PAs negatively impact clinical outcomes, and 94% reported that PAs were associated with delays in necessary care for those patients whose treatment required PA.16 Moreover, 33% of physicians surveyed reported that a PA issue had led to a serious adverse event for their patient(s), including hospitalization, a life-threatening event, or disability and permanent bodily damage.16 Roundtable participants shared that the ability to leverage an electronic PA (ePA) has helped to align resources and decrease administrative burden, but ePAs may not be as immediately and universally effective based on the rapid changes happening within the management of acute leukemias.
The Need for Utilization Management Policy Reform
Because of the large burden the PA process places on institutions and provider organizations, providers are calling for reform and the creation of new utilization review policies. The results of a 2017 survey of 394 hematology and oncology practices (representing 58% of the US hematology and oncology workforce) showed that 58% of respondents identified payers as the primary source of strain on their practice. The survey identified PAs at the top of the stressors, which also included staffing issues, drug pricing, and increasing practice expenses.17
Several organizations, such as the American Society of Clinical Oncology (ASCO), recognize the burden of PAs on institutions and practices and are advocating for streamlined PA processes to stop delays in care and reduce administrative burden. In January 2017, the AMA and 16 other professional organizations jointly urged an industry-wide reassessment of PA programs. The aim of the initiative was to align PA programs with a newly created set of 21 principles designed to ensure that patients receive timely and medically necessary care and to reduce administrative burdens. These 21 principles were classified under 5 categories: 1) clinical validity, 2) continuity of care, 3) transparency and fairness, 4) timely access and administrative efficiency, and 5) alternatives and exemptions.18
The topic of clinical and cost burdens associated with PA processes was escalated to Washington, DC, garnering attention from the US Congress. In September 2022, the House of Representatives passed the Improving Seniors’ Timely Access to Care Act of 2022. The bill calls for the implementation of real-time decisions about PAs, requires the Centers for Medicare & Medicaid Services (CMS) to report the rate of PA approvals and denials, and urges plans to follow evidence-based guidelines when consulting with physicians over PA requests.19
Guideline-Concordant Care: An Evidence-Based Review Processes for Decision-Making
Payers and physicians often draw treatment recommendations from published and peer-reviewed guideline advice. Guideline-concordant care is the management of conditions that is consistent with treatment approaches defined in these peer-reviewed guidelines, such as the NCCN.20 Payers will generally align formulary and coverage policies with these guideline-recommended approaches for the best standard of care, as well as nationally recognized compendia such as The NCCN Drugs and Biologics Compendium®.21
Payer decision-making processes for rare hematologic cancers are challenging in comparison with those for more prevalent and widely studied malignancies. For example, coverage decisions for high-prevalence cancers such as lung, prostate, or breast cancers generally are based on clinical efficacy and safety data from large randomized clinical trials (RCTs), real-world evidence (RWE), guidelines, and cost-effectiveness data.20
When making initial coverage decisions for new therapies, payers also review FDA-approved labeling and professional guideline recommendations and other published compendia and consult expert panels within their organizations. When considering the extension of coverage to new indications, payers may also review scientific literature to identify publications describing emerging treatment approaches.
In rare conditions with limited effective treatment options (for example, subtypes of ALL), roundtable participants agreed that payers and clinicians need to weigh the strength and depth of available evidence against the acuity of the disease and the urgency for treatment initiation. Payers review FDA labeling, NCCN Guideline recommendations, and other disease- and treatment-specific compendia as support in determining if the treatment requested is approvable from an insurance coverage standpoint. They also review peer-reviewed scientific literature to extend coverage, off-label at times. Payers confirm whether a therapy is the best fit for that patient and their unique situation and look at the totality of the disease, the phase/stage, and line of therapy. According to Bobolts, “There are [few] options for acute leukemias, so the most valuable therapy for the patient is [essentially] predetermined. [Therefore], in that setting, when you’re looking at value, you’re looking at everything at your fingertips that you can grab.” She added that for patients with these acute conditions, some of the more desirable data, such as RCTs with large sample sizes, health-related quality of life studies, and cost-effectiveness analyses, may not be available.
Participants shared that there is a need for concordance with clinical practice guidelines (eg, NCCN). Some participants cited prior success with aligning NCCN Guidelines with PA criteria, which then helped to create regimens that could be automatically approved rather than regimens that required custom PA creation. Aligning oncology PA criteria with NCCN Guidelines and using auto-approval to expedite PAs can streamline access and reduce payer administrative costs and barriers associated with custom PA criteria development for each new oncology therapy.
Clinical and Cost Benefits of Guideline-Concordant Care
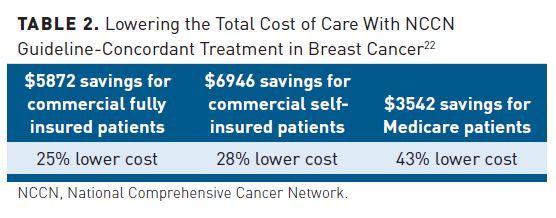
Recently published studies have examined the relative quality and cost-effectiveness of guideline-concordant and nonconcordant treatment. The results of a retrospective database study of over 300 patients with breast cancer showed over 40% savings in the total costs of care (TCOC) per member, per month (PMPM) when guideline-concordant treatment is utilized in Medicare populations (Table 2).22 Differences were evident in provider chemotherapy spend and outpatient spend.
Results from a similar study of 937 patients with colon cancer found a significant reduction (33%) in PMPM TCOC for patients in the Medicare group who received guideline-concordant treatment compared with matched controls who received nonconcordant treatment (P < .001). This difference was driven primarily by medical chemotherapy spend.23 Both studies highlight the importance of evidence-based guidelines in delivering value-based cancer care.
In June 2015, UnitedHealthcare implemented a mandatory PA program for an injectable chemotherapy prescribed for members who were commercially insured. The program was designed to improve quality of care and reduce service denials by encouraging providers to offer guideline-concordant treatments. Using an online PA tool, providers are asked to submit the minimal amount of information about a patient’s disease characteristics to generate treatment recommendations based on the NCCN Guidelines. The PA program integrates real-time decision support that can minimize authorization denials and lower the total cost of chemotherapy treatments using a digital system from eviCore.24
During the 1-year pilot of the UnitedHealthcare program in a Florida commercial plan population, 4272 eligible cases were reviewed, and only 42 (1%) were denied. Immediate online approvals were obtained for 58% of cases without any further interaction, and approval was granted for 95% of the remaining cases within 24 hours. The study results also showed a 20% decrease in cost of chemotherapy drugs, representing estimated drug savings of $5.3 million. Findings from this study have important policy implications, highlighting how computer-based PA methods may lower costs to offset financial burdens of newer and often more effective treatments.25
Rare Hematologic Cancer Case-Study: Ph+ ALL Treatment Landscape in the Frontline Setting
Guideline-concordant care approaches for Ph+ ALL in adult patients vary depending on the ALL variant and the presentation of disease.2 First-line, induction-phase, NCCN-concordant therapy for Ph+ ALL outside of a clinical trial includes a tyrosine kinase inhibitor (TKI) in combination with corticosteroids, chemotherapy, or blinatumomab. Reinduction therapy for relapsed/refractory Ph+ ALL includes, among other options, TKI used in combination with other induction therapies that patients have not yet received. TKI options listed in the NCCN Guidelines for first-line therapy of Ph+ ALL include, in alphabetical order, bosutinib, dasatinib, imatinib, nilotinib, and ponatinib. Selection of a first-line TKI for Ph+ ALL depends, in part, on data within the context of the specific regimen being considered, as well as the age and comorbidities of the patient. In patients with known mutations in the kinase domain of BCR-ABL, such as T315I, selection of a TKI should also incorporate this information.26
The presence of the T315I mutation in patients with Ph+ ALL is associated with increased cumulative risk of relapse, with a median time to relapse of 7 months and shorter overall survival (OS).27 Ponatinib (ICLUSIG®; Takeda Pharmaceuticals U.S.A., Inc) is a BCR-ABL–targeted therapy that has been FDA-approved for patients with T315I-positive Ph+ ALL and patients with Ph+ ALL for whom no other TKIs are indicated based on contraindications; it is NCCN-endorsed in both the frontline and relapsed/refractory settings as part of therapy for Ph+ ALL.28
The introduction of BCR-ABL–targeted therapies has substantially increased survival in patients with Ph+ ALL, and it plays a leading role in the prevention of treatment resistance.8,29 Between 1984 and 1989, the 3-year survival rate for Ph+ ALL was 5%. In 2000, when targeted TKIs were first introduced, OS rates rose from 15% to 48% and continued to trend upwards, reaching 79% through 2019.30
Results of investigational studies show that novel combinations of TKI and selective antibodies are highly effective in treating frontline Ph+ ALL. Since 2018, the use of potent combination regimens, such as hyper-CVAD (hyperfractionated therapy consisting of cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, and dexamethasone + methotrexate and cytarabine) plus ponatinib, or blinatumomab plus dasatinib or ponatinib, has contributed to longer survival in patients with Ph+ ALL.30
Oncologists have been incorporating combination therapies with advanced-generation TKIs in earlier lines of treatment with the goals of suppressing resistance mechanisms, reducing the risk of relapse, and potentially reducing the number of patients who warrant allogeneic stem cell transplantation or chimeric antigen receptor T-cell therapy or who require hospitalizations for treatment-related complications. Geyer explained the important role of TKIs, such as ponatinib, in the frontline setting: “Everyone can agree that we’ve moved the needle significantly by incorporating more potent TKIs in the frontline setting,” he said. “By using a more potent TKI like ponatinib in the frontline setting, we are seeing fewer relapses down the line, to the point where ponatinib is one of the TKIs that’s allowed in the Eastern Cooperative Oncology Group (ECOG) RCT of chemo vs chemo-sparing–based therapy in combination with that potent TKI.” Appropriate use of TKI combination therapies in Ph+ ALL may help lower health care resource utilization costs by preventing hospitalizations.
Streamlined Solutions to Challenges for Ph+ ALL Expedited Access to Therapy
Key stakeholders participating in the roundtable discussed possible streamlined solutions to expedite access to therapy that could apply to patients with Ph+ ALL. Proposed solutions included: 1) the use of ePA and other digital solutions that enhance communication between payers and providers, 2) expedited approval pathways for use in specific conditions, 3) the use of RWE for decision-making, 4) the issuing of PA “Gold Cards,” and 5) a shift to value-based care agreements.
Digital Solutions to Streamline the PA Process
Payer participants acknowledged the barriers and downsides of PA processes. They agreed that they actively work with providers in their plan to reduce any pain points PAs can cause. “Provider experience is very important in our plan,” explained Tar. “It is a top priority for the pharmacy department as well as an organizational goal.”
Experts from the roundtable also discussed PA program characteristics that can improve access to care and outcomes for patients with acute oncology care needs. These concepts were explored through a case study using the Transform Oncology Care (TOC) program at CVS Health. Participants discussed how concepts gleaned from the program could be implemented in other organizations. Notable key features that were highlighted included linking the program with an electronic health record (EHR) system, alignment with NCCN-concordant management, and a PA approval process that follows patients from diagnosis to care and maintenance.
Bobolts and others shared that payers value programs that look at multiple touchpoints throughout the patient journey rather than individual PAs to help patients access therapy. Although the CVS TOC program has good attributes, payers noted there is uncertainty around how this solution is being adopted within the limitations of EHR integration and data sharing. Payers shared that they are working on similar ideas in their organizations to reduce provider burden. Bobolts noted that “there are challenges with sharing data. [These challenges can include] an EHR company that is not willing to build an application programming interface to share data, the sheer volume of different EHRs that a payer would need to integrate with, and gaining provider approval to extract data on demand from an EHR.”
ePA and EHR Integration
An ePA for pharmacy benefit drugs (ie, oral TKIs or oral chemotherapy) is available in many of the most widely used EHR systems, including those offered by Cerner and Epic. Surescripts and CoverMyMeds are some of the currently available ePA online tools for providers; they are designed to integrate clinical information required for PAs into EHR systems. There is an opportunity to use Surescripts or other ePA tools that utilize e-prescribing across several different centers. However, the key is integration among systems, and these tools may have a limited capacity to ePA medical benefit drugs (ie, injectable therapies such as chemotherapy or intravenous targeted therapies) and may not be able to authorize a multidrug cancer treatment regimen in 1 authorization, requiring multiple data entries. Without ePA integration into existing EHRs for all cancer drugs at the regimen level, irrespective of medical or pharmacy benefit, providers must use different ePA systems for each insurance provider, creating an additional burden on practices.
There are concerns regarding ePA technology customization and provider burden. One roundtable participant cited a challenge in the technology as to how these systems are created. Tar shared that a small proof-of-concept study was launched at her health plan (Blue Cross Blue Shield of Massachusetts) with a local specialty hospital.31 By leveraging an existing system the hospital had in place through Olive, a health tech company with artificial intelligence (AI) capabilities, the health plan was able to integrate PA requirements and allow the system to search for the information needed to complete the medical necessity review for each case.
The piloted program delivered necessary information to the provider, including PA requirements. Results of the proof of concept demonstrated a streamlined PA process, reduced denial rates, and allowed for real-time decisions. A key finding of the pilot study was that providers were submitting PAs for treatments that did not require PA approvals. By incorporating the health plan requirements into the Olive system, the proof-of-concept program was able to alert providers as to which treatments required a PA and which did not. If PA was required and the AI did not find all the information it needed, the case would be pended and sent to a nurse to obtain more information. Tar noted that, although successful, the pilot was not initiated in oncology, an overtly complex disease state.
Evolution in EHR and ePA technologies are widely anticipated. Although EHR companies and online ePA support systems such as CoverMyMeds have revolutionized access to products covered by pharmacy benefits, oncology patients need similar support tools for therapies covered under the medical benefit. Current EHR systems are often not capable of managing patients across both the medical and pharmacy benefit, assisting with the PA for more than 1 medical benefit product at a time, or handling regimen-based authorizations.
A potential obstacle to improving ePA technology is ensuring EHR systems align with NCCN-concordant care, which helps providers ensure that they are delivering the best standard of care. EHRs must use a standard nomenclature, such as International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) or ICD-10-Procedural Coding System (ICD-10-PCS) diagnosis codes, to achieve the level of precision required by molecular medicine.
Leverage Data Sharing With EHR
Identifying ways to more efficiently share data may help to increase approval rates and improve the PA submission process. EHR integration varies by the specific interface being used, and configuration or customization of individual provider technologies may limit or hinder the ability to standardize the experience.
Haumschild discussed his experience with Epic integration in his institution and how it streamlined the PA process. Organizations such as Flatiron are trying to aggregate quality real-world data to help support prescriber decisions. Creating a system in which prescribers can review evidence-based information on different treatment pathways may facilitate optimal care and expedited approval. “When you submit your medication into the EHR, you immediately receive the ePA to fill out and submit,” he explained. “When the EHR is being used as dispensing software in the pharmacy, we have it as the medical benefit. [This integration] creates streamlined opportunities, reduces burden, allows for more transparency, and there is a timelier response. Partnering with a third-party to share information in a nonbiased way allows for an efficient transmission.”
If the treatment center is not providing their own solution or treatment pathway, there should be the opportunity to work with the large EHR platforms (ie, Cerner or Epic) and allow vendors that already have that solution to be able to provide access to it. One challenge is that provider-based clinical oncology pathways, or decision support embedded into EHRs, lack connectivity with a payer’s PA process. This disjointed process can become more complicated if a provider uses oncology pathways that differ from a payer’s pathways or if the various payers the provider works with most often also use different pathways.
Roundtable participants identified several reasons why it may be helpful to incentivize an EHR to be integrated with payers in areas such as data generation and ease of access to appropriate medications that are consistent with evidence-based guidelines. EHR integration and connectivity would ensure that payers have access to the information needed for PA approval, thus potentially expediting authorizations, reducing barriers to access, and drastically reducing denials that occur from a lack of vital information.
Improved Communication Regarding PAs
To date, many ePAs have not yet been fully optimized to handle complex clinical care, such as when patients require multiple medications simultaneously. Bobolts explained that her organization’s ePA process is at the regimen level, allowing authorization of all cancer treatment and supportive care drugs to be addressed within a single ePA of medical and pharmacy benefits together (instead of 5 separate authorizations for 5 different treatments).
In addition, the use of ePA may not be as widespread as it could be. In an analysis of the data available on ePA use, Bobolts found that only 44% of practices were utilizing ePA, whereas 40% submitted the PA via email or eFax, 14% submitted through a phone call, and 2% mailed PAs. She added, “Services are available, and people need to take advantage of tools like ePA that are provided to reduce the burden on providers.”
Improved communication between payers and providers is also important for urgent patient cases, and it can be an efficient way of gaining PA approval. Payer and provider participants agreed that improved communication can streamline the PA approval process, even for complex cases. The sharing of additional clinical details and data found within the EHR system can be useful in rare diagnoses like Ph+ ALL.
Payer and provider participants identified obstacles that could potentially be overcome through improved real-time communication. These included avoiding the time-consuming back and forth between providers and payers via eFax or submission of denials via ePA and days spent waiting for follow-up to identify missing information. Without direct communication with the PA case contact, it can take additional time to find the information needed to resolve the case.
Alternative Expedited Approval Pathway for Urgent PA
Bobolts advocated that there is a need to revolutionize and modernize PA by automating approvals and addressing barriers to timely care interventions. In Ph+ ALL and other rare diseases with few FDA-approved frontline treatments, there is a need for PA processes that can respond to treatment urgency. Stakeholders from the roundtable agreed that requests for these patients need expedited review. Geyer proposed that for certain medications in some of the acute settings, the payer should provide an initial 30-day approval so that patients have access to medication quickly while providers and payers work together to arrange long-term coverage. However, from a payer’s perspective, an initial approval without verification of medical necessity may create obstacles in the management of long-term coverage after treatment has already begun. Tar and Geyer shared that patients would benefit from a process that flags certain ICD-10-CM codes with urgency, automatically triggering an expedited review. For example, a diagnosis code for Ph+ ALL is implicit of urgency and would elicit an expedited review.
Additional risks exist surrounding PA criteria for rare diseases. Geyer noted that PA criteria for rare cancers may be infrequently reviewed, and because the affected population is so small, it could lead to denial of guideline-concordant care. He also noted that in some cases, the peer reviewer of the PA criteria at the payer level may have little or no experience in managing the condition.
Participants agreed there is a need for implementing a real-time PA solution. Bobolts provided an example from her organization, which provides oncology-specific PAs for ponatinib for roughly 15 health plans. “Our turnaround time was 5.13 hours to make the decision of approval on the prior authorization for treatment. And for maintenance, it was 2.84 hours,” she said. She explained that although this turnaround time was expedited, it may not be reflective of all oncology cases, and it was only able to occur because the organization was aware that a fast response is required for patients with ALL and other acute leukemias. “Providers need a decision as soon as possible,” said Bobolts. “For maintenance [treatment], typically, most of the patient’s records are available from the earlier authorizations, so if the payer is looking to see if the patient has T315I [mutation] or not, the information is readily available. If not, they can request it. But usually, those can be faster subsequent authorizations for patients.”
Tar added that there may be a gap in how utilization management decisions are driven between the pharmacy benefit vs the medical benefit, because on the pharmacy benefit, medications that may have an urgent need would typically not have a utilization management edit. This is because the burden of potentially hindering access to urgently needed care outweighs the value of adding a utilization management edit. In her system, oncology reviews are outsourced to Carelon Insights (formerly AIM Specialty Health), which follows NCCN Guidelines for reviews. The discussion among participants raised the question about whether, for certain severe diseases, there may be instances where treatments could bypass PA requirement.
Opportunities for Leveraging Real-World Evidence to Support Coverage Decisions
The utility of payer data to track patient treatments and outcomes, thus providing future guidance and optimization of PA decisions, is restricted due to the limited duration of patient membership in each health plan. Generally, a lack of connectivity between PA decisions and patient outcomes limits the ability of payers and health systems to accurately correlate PA decisions with patient-level outcomes.
For example, provider and payer participants agreed that the lack of data—both peer-reviewed and real-world—contributes to the challenges in timely PAs for rare disease states including rare leukemias. Universal adoption of off-label coverage deemed medically acceptable according to eligible compendia, peer-reviewed scientific literature, or standards published by CMS for Medicare patients, could streamline access for smaller patient populations, irrespective of line of business.32
Although real-world data can be obtained from patients who are treated in academic centers, these data are not always published in a top-tier, high-impact journal (ie, Blood or NEJM), which would make them eligible by CMS for off-label coverage. According to Geyer, “If we’re going to accept real-world evidence, we also need to say, ‘If it’s peer-reviewed real-world evidence that reaches a certain standard, we can actually use this as another piece of data to support a coverage decision in some cases.’”
For smaller patient populations (eg, those with a rare, acute leukemia), leveraging RWE may be possible for decision-making in lieu of randomized clinical trials. However, Bobolts explained that in the payer landscape, payers generally follow the well-established Medicare criteria for reviewing data for off-label coverage.32 Participants agreed that quality of RWE for coverage support is an area that needs to be explored, as there is not enough evidence that is: 1) published or 2) published in 1 of the 26 CMS-supported journals where it can be leveraged for off-label approval. “Providers need to generate data on therapy that has real-world evidence that shows benefit and is published in an appropriate [CMS-supported] journal so that we can justify coverage,” said Bobolts. “Even if the data aren’t published in the appropriate journal, [generate it] anyway so that the payer can have some clinical justification that the off-label therapy is [the] best fit for the patient. We need something tangible to try to justify coverage support and will fight tooth and nail with Medicare should it be audited. Do it for the patient.”
Roundtable participants agreed that additional RWE is needed to support patients whose cancer presentation does not fall precisely within existing NCCN Guidelines, such as those with rare disease presentation or uncommon mutation variants. When a patient falls into a clear NCCN-concordant patient group, PA approvals for appropriate therapies are more straightforward. When a patient’s disease presentation falls outside treatment guidelines, payers need to search for scientific literature to help facilitate an expedited coverage determination.
Bobolts added, “A payer is not likely to deny therapy if it is supported by guidelines or compendia, such as [from] the NCCN. We are bound by regulations. The last thing a payer wants to do is issue an inappropriate denial because they failed to notice new groundbreaking scientific advances since the PA criteria was developed, putting a barrier in the patient’s way to accessing the latest high-quality cancer care. We also need to look beyond the NCCN at times and say, ‘If something hasn’t made it into NCCN, what else can we incorporate to break down barriers and extend authorization?’”
Prior Authorization “Gold Cards”
PA “Gold Carding” is another potential solution for a select number of eligible providers who are practicing according to preselected guidelines. This method, which has been outlined in HR 7995, Getting Over Lengthy Delays in Care as Required by Doctors Act of 2022, and is state law in Texas and a small number of other states, proposes PA exemption to providers or practices that have a certain percentage of PA requests approved within the prior plan year.33 Practices granted an exemption would be assigned a “Gold Card,” a name that is not finalized and that may need to be updated with a more industry-neutral name.33
However, these laws have limitations. During the roundtable event, participants cited provider confusion regarding Gold Carding laws and reimbursement, and they noted the potential for delays in patient care or the disadvantage this places on those patients who are not under the care of a Gold Card provider.
Value-Based Care Arrangements
In a value-based contracting arrangement, payers collaborate with high-performing providers who deliver high-quality, cost-effective care. This model is a shift from the fee-for-service model to one that pays for the value of therapy provided.
These value-based care models, most of which are still in development, provide an innovative solution, but they may require a provider to accept more financial risk. According to the payer roundtable participants, this model would require “willing partners,” as a provider would need to be willing to be paid for the value of a therapy to an individual patient rather than the quantity of therapy prescribed over their entire patient population.
In this model, providers may be required to take on some financial risks to offset the potential lost affordability for health plans. “Moving more providers to value-based contracting represents a long-term solution to reduce provider burden in the first place,” said 1 roundtable participant. Bobolts agreed that payers are more interested in developing value-based care programs that support high quality, cost-effective care. “We are hopefully shifting in that direction in the coming years,” she said. “We need people to be forward-thinking to try to pay for the value therapy brings first, not just the quantity of therapy prescribed.”
Conclusions
The purpose of utilization management is to help health care systems and payers manage costs and reduce waste while ensuring that patients get medically appropriate care in the correct setting. Yet some utilization management processes, such as PAs, can create barriers to access for patients. There is a need for the development of technology and processes that alleviate provider burden. Such goals may be achievable through the enhancement of the PA process, the use of ePAs to expedite patient care initiation, the adoption of a platform that offers interoperability with EHR systems, and the enhanced ability to share data to expedite authorizations and support utilization and cost analyses. Additional utilization management tools and processes are needed to aid in the treatment of rare diseases, where peer-reviewed and real-world data tend to be more finite.
Although potential solutions to streamlining the PA process have been proposed and integrated within certain organizations, most proposed solutions represent short-term fixes. A potential long-term solution preferred by payer participants would be moving toward a value-based care system. In rare diseases such as Ph+ ALL, where a quick initial start to treatment is critical, a value-based care approach might make provider reimbursement contingent on the quality of care provided, and PAs could potentially be waived for providers with a high rate of quality care outcomes. Improved communication between providers and payers is critical for the timely approval of PAs and for patients to get appropriate care in a timely manner. Early and frequent communication during the PA approval process will help alleviate any concerns and create clarity on the process, improving the timeliness of patient care.
Disclaimer: Opinions expressed by the authors are their own and do not necessarily represent those of their respective employers.
Author Affiliations: The University of Texas MD Anderson Cancer Center (EJJ), Houston, TX; OncoHealth (LRB), Plantation, FL; United HealthCare Services, Inc. (TES), Edina, MN; Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (MBG), New York, NY; Blue Cross Blue Shield of Massachusetts (VTET), Boston, MA; Winship Cancer Institute (RH), Atlanta, GA.
Funding Source: This supplement was supported by Takeda Pharmaceutical Company.
Author Disclosures: Dr Jabbour is employed by The University of Texas MD Anderson Cancer Center and reports providing expert testimony for AbbVie, Amgen, Ascentage Pharma Group, Hikma Pharmaceuticals, Kite Pharma, and Takeda. Ms Spinks is employed by United HealthCare Services, Inc., and reports an institutional conflict of interest due to her employer’s utilization of a prior authorization process for chemotherapy. Dr Geyer reports serving as an advisory board member for Novartis and Sanofi. He also reports receiving research support from Actinium Pharmaceuticals, Amgen, and Sanofi. Dr Bobolts, Dr Ebot-Tar, and Dr Haumschild report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (EJJ, LRB, MBG); acquisition of data (EJJ, TES, MBG); analysis and interpretation of data (EJJ, MBG, RH); drafting of the manuscript (EJJ, LRB, TES, RH); critical revision of the manuscript for important intellectual content (EJJ, LRB, TES, MBG, VTET, RH); and subject matter contribution (VTET).
Address Correspondence to: Ryan Haumschild, PharmD, MS, MBA. Email: Ryan.Haumschild@emoryhealthcare.org
References
- National Cancer Institute. Dictionary of cancer terms – rare cancer. Accessed February 8, 2023. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/rare-cancer
- Abunadi I, Senan EM. Multi-method diagnosis of blood microscopic sample for early detection of acute lymphoblastic leukemia based on deep learning and hybrid techniques. Sensors (Basel). 2022;22(4):1629. doi:10.3390/s22041629
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer stat facts: leukemia—acute lymphocytic leukemia (ALL). Accessed February 9, 2023. https://seer.cancer.gov/statfacts/html/alyl.html
- Cancer facts & figures 2023. American Cancer Society. Accessed February 8, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Shi T, Huang X, Zhu L, Li X, Li L, Ye X. Adult Ph-positive acute lymphoblastic leukemia-current concepts in cytogenetic abnormalities and outcomes. Am J Cancer Res. 2020;10(8):2309-2318.
- Ronson A, Tvito A, Rowe JM. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia. Curr Treat Options Oncol. 2017;18(3):20. doi:10.1007/s11864-017-0455-3
- PH-positive ALL therapy. Leukemia & Lymphoma Society. Accessed March 10, 2023. https://www.lls.org/leukemia/acute-lymphoblastic-leukemia/treatment/ph-positive-all-therapy
- Ravandi F. Managing Philadelphia chromosome-positive acute lymphoblastic leukemia: role of tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk. 2011;11(2):198-203. doi:10.1016/j.clml.2011.03.002
- Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653-661. doi:10.3324/haematol.2014.118588
- Musleh S, Islam MT, Alam MT, Househ M, Shah Z, Alam T. ALLD: Acute lymphoblastic leukemia detector. Stud Health Technol Inform. 2022;289:77-80. doi:10.3233/SHTI210863
- Brown PA, Shah B, Advani A, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(9):1079-1109. doi:10.6004/jnccn.2021.0042
- Barata A, Wood WA, Choi SW, Jim HSL. Unmet needs for psychosocial care in hematologic malignancies and hematopoietic cell transplant. Curr Hematol Malig Rep. 2016;11(4):280-287. doi:10.1007/s11899-016-0328-z
- El-Jawahri A, Nelson AM, Gray TF, Lee SJ, LeBlanc TW. Palliative and end-of-life care for patients with hematologic malignancies. J Clin Oncol. 2020;38(9):944-953. doi:10.1200/JCO.18.02386
- Giardino AP, Wadhwa R. Utilization management. In: StatPearls. StatPearls Publishing. Updated January 2023. National Center for Biotechnology Information. Accessed March 29, 2023. https://pubmed.ncbi.nlm.nih.gov/32809641/.
- 2018-2019 Academy of Managed Care Pharmacy Professional Practice Committee. Prior authorization and utilization management concepts in managed care pharmacy. J Manag Care Spec Pharm. 2019;25(6):641-644. doi:10.18553/jmcp.2019.19069
- American Medical Association. 2022 AMA prior authorization (PA) physician survey. Accessed
March 23, 2023. https://www.ama-assn.org/system/files/prior-authorization-survey.pdf - Kirkwood MK, Hanley A, Bruinooge SS, et al. The state of oncology practice in America, 2018: results of the ASCO Practice Census Survey. J Oncol Pract. 2018;14(7):e412-e420. doi:10.1200/JOP.18.00149
- American Medical Association. Prior authorization and utilization management reform principles. Accessed March 22, 2023. https://www.ama-assn.org/system/files/principles-with-signatory-page-
for-slsc.pdf - Improving Seniors’ Timely Access to Care Act of 2021, HR 3173, 117th Congress (2022). Accessed March 29, 2023. https://www.congress.gov/bill/117th-congress/house-bill/3173.
- National Comprehensive Cancer Network (NCCN). Development and update of guidelines. Accessed March 22, 2023. https://www.nccn.org/guidelines/guidelines-process/development-and-update-of-guidelines
- NCCN drugs & biologics compendium. National Comprehensive Cancer Network. 2023. Accessed March 23, 2023. https://www.nccn.org/compendia-templates/compendia/drugs-and-biologics-compendia
- Sapkota U, Cavers W, Reddy S, Avalos-Reyes E, Johnson KA. Total cost of care differences in National Comprehensive Cancer Center (NCCN) concordant and non-concordant breast cancer patients. J Clin Oncol. 2022; 40(suppl 16):abstr e18833. doi:10.1200/JCO.2022.40.16_suppl.e18833
- Sapkota U, Cavers W, Reddy S, Avalos-Reyes E, Johnson KA. Total cost of care differences in National Comprehensive Cancer Center (NCCN) concordant and non-concordant patients with colon cancer. J Clin Oncol. 2022;40(suppl 16):3624. doi:10.1200/JCO.2022.40.16_suppl.3624
- Byfield SD, Chastek B, Korrer S, Horstman T, Malin J, Newcomer L. Real-world outcomes and value of first-line therapy for metastatic non-small cell lung cancer. Cancer Invest. 2020;38(10):608-617.
doi:10.1080/07357907.2020.1827415 - Newcomer LN, Weininger R, Carson RW. Transforming prior authorization to decision support. J Oncol Pract. 2017;13(1):e57-e61. doi:10.1200/JOP.2016.015198
- NCCN. Clinical Practice Guidelines in Oncology. Acute lymphoblastic leukemia, version 2.2022. Accessed March 23, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Short NJ, Kantarjian H, Jabbour E. SOHO state of the art updates & next questions: intensive and non-intensive approaches for adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(2):61-66. doi:10.1016/j.clml.2021.08.003
- Iclusig. Prescribing information. Takeda Pharmaceuticals America, Inc; 2022. https://www.iclusig.com/sites/default/files/2023-02/iclusig-prescribing-information.pdf
- Nicolini FE, Mauro MJ, Martinelli G, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR-ABL T315I mutation. Blood. 2009;114(26):5271-5278. doi:10.1182/blood-2009-04-219410
- Jabbour E, Haddad FG, Short NJ, Kantarjian H. Treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemia-from intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 2022;8(9):1340-1348. doi:10.1001/jamaoncol.2022.2398
- Blue Cross Blue Shield of Massachusetts uses artificial intelligence to speed review time, automate authorizations & eliminate administrative costs. Blue Cross Blue Shield of Massachusetts. October 12, 2022. Accessed March 8, 2023. https://newsroom.bluecrossma.com/2022-10-12-blue-cross-blue-shield-of-massachusetts-uses-artificial-intelligence-to-speed-review-time,-automate-authorizations-eliminate-administrative-costs
- Medicare benefit policy manual chapter 15 – covered medical and other health services. US Department of Health & Human Services. July 12, 2019. Accessed March 23, 2023. https://www.hhs.gov/guidance/document/benefit-policy-manual-chapter-15-covered-medical-and-other-health-services
- Getting Over Lengthy Delays in Care as Required by Doctors Act of 2022 or the GOLD CARD Act of 2022, HR 7995, 117th Congress (2022). June 9, 2022. Accessed March 23, 2023. https://www.congress.gov/bill/117th-congress/house-bill/7995?s=1&r=88
