- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Patient Well-being and the Clinical and Economic Burdens Associated With Obesity in the United States
ABSTRACT
Obesity is a serious, progressive, chronic disease that is associated with a spectrum of complications and poor outcomes (eg, premature death, diminished quality of life) and is a risk factor for several other diseases. Obesity increases the risk of developing type 2 diabetes, cardiovascular disease, and certain cancers. More recently, obesity was recognized as a risk factor for poor outcomes in patients with COVID-19. When experienced concurrently with a serious disease, obesity may increase the risk of negative health outcomes. Furthermore, individuals with obesity are more likely to experience social stigma and discrimination at work and in educational and health care settings; these may impact mental and physical health and contribute to increased adiposity. In the United States, the economic burden of obesity is immense—according to estimates, hundreds of billions of dollars are spent annually on direct medical needs and lost productivity associated with obesity. More severe classes of obesity greatly impact both the health of individuals and health care expenditures. As obesity becomes more prevalent, policy makers, health care professionals, and payers must consider its clinical, social, and economic implications.
Am J Manag Care. 2022;28(suppl 15):S279-S287. doi:10.37765/ajmc.2022.89291
For author information and disclosures, see end of text.
Introduction
The prevalence of obesity among US adults has steadily increased since the late 1970s, growing from 15.0% in 1976-1980 to 42.8% in 2017-2018 among those aged 20 to 74 years.1,2 Defined by a body mass index (BMI) of at least 30 kg/m2, obesity is the most substantial risk factor for chronic disease in the United States and is associated with nearly 200 related complications.3-7 The 2016 American Association of Clinical Endocrinology/American College of Endocrinology obesity treatment guidelines clarified that, as part of a larger diagnostic staging process, overweight and obesity should be assessed not just by BMI, but also by measurement of waist circumference, patient examination, and clinical interpretation of weight-related comorbidities.3 In the third article of this supplement, Marc-Andre Cornier, MD, discusses the guideline-recommended evaluation, classification, and treatment of overweight and obesity in adults.8 This article explores the patient and health care resource burden of obesity and certain related comorbidities (eg, metabolic disorders, cardiovascular disease [CVD], some cancers) that pose the greatest burden on global health.7 The escalating prevalence of obesity may be the precursor for future clinical, well-being, and economic burdens.
Clinical Burden of Obesity
Some of the most notable complications either caused or exacerbated by excess adiposity include type 2 diabetes (T2D), CVD, dyslipidemia, hypertension, polycystic ovary syndrome, female infertility, male hypogonadism, obstructive sleep apnea, asthma, osteoarthritis, depression, gastroesophageal reflux disease, nonalcoholic fatty liver disease, and urinary stress incontinence.3 Although a comprehensive description of the relationships between obesity and the full array of related comorbidities is beyond the scope of this article, the following describes several common and prominent comorbidity clusters that exert a substantial clinical burden upon individuals with obesity.
Cardiometabolic Disease
Cardiometabolic disease describes a group of related cardiovascular and metabolic disorders; these include T2D, dyslipidemia, hypertension, coronary artery disease (CAD), venous thromboembolic disease, and stroke.3,9,10 The mechanistic relationships between obesity, insulin resistance, and cardiometabolic disease are not yet fully understood. Still, obesity can be associated with increased insulin resistance, and both obesity and weight gain are associated with poor glucose control in patients with T2D.3 Waist circumference, abdominal obesity, and ectopic adiposity are associated with CVD independent of BMI; furthermore, obesity and increased BMI are associated with overt atherosclerotic lesions, amplified risk of atrial fibrillation, incident CAD, venous thromboembolism, pulmonary embolism, and sudden cardiac death.9-12 The risk factors for CVD in obesity function in complex, interrelated, and bidirectional relationships.13 For example, the pathophysiologic mechanisms related to development of hypertension via endothelial dysfunction—including inflammation, oxidative stress, and activity of adipokines (ie, cytokines secreted by adipose tissue) and the renin-angiotensin-aldosterone system—also are linked to diseases affecting cardiovascular, cerebrovascular, and renal function, and they can be exacerbated by increases in adiposity.3,10,13,14 Excess weight, and particularly visceral adiposity, is the principal cause for 60% to 70% of adult hypertension.3 In addition, abnormal adiposity may induce insulin resistance, which is associated with the cardinal characteristics of dyslipidemia: increased plasma triglyceride, total cholesterol, and low-density lipoprotein (LDL) cholesterol levels and reduced high-density lipoprotein (HDL) cholesterol levels.3,9,10
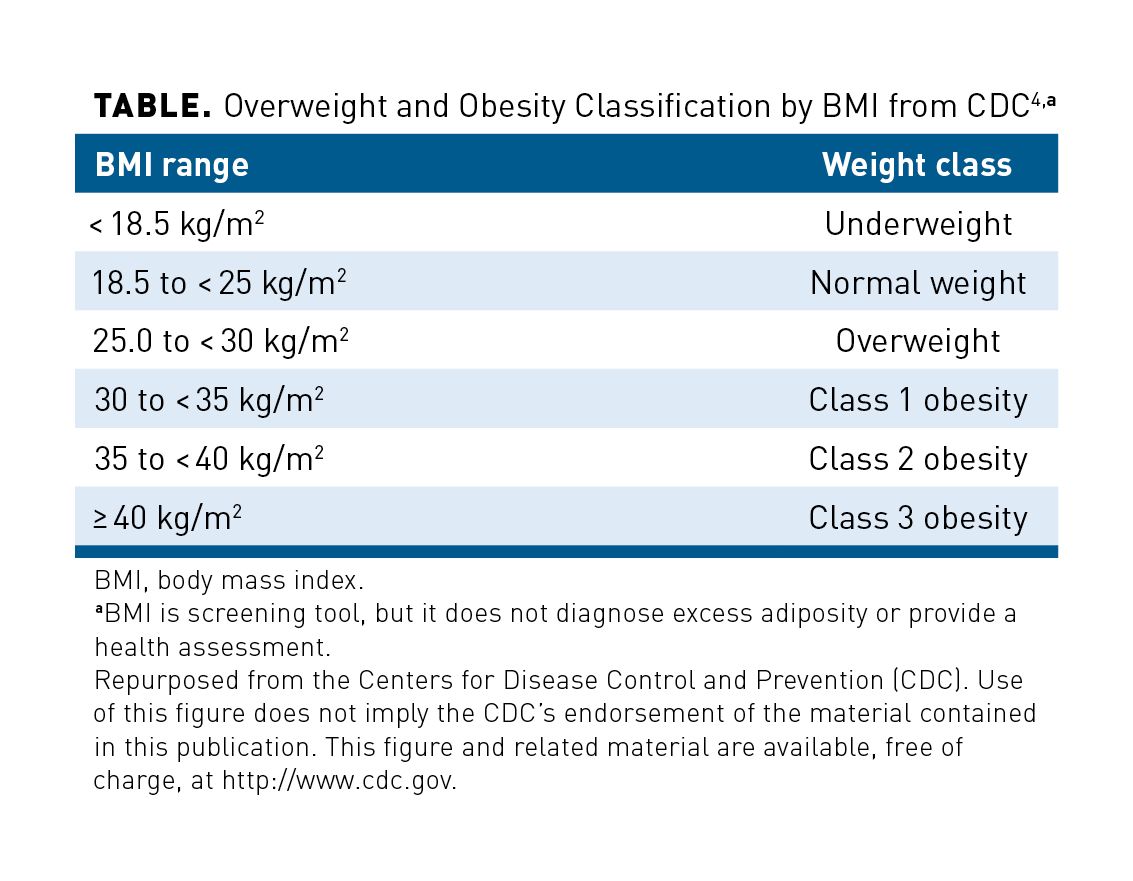
BMI ranges are used to categorize weight into classes from underweight through class 3 obesity (Table).3,4 In a study published in 2015, investigators developed a model from National Health and Nutrition Examination Survey (NHANES) data (2003-2010) to compare estimates of life expectancy in non-Hispanic White US adults; data extracted from US national life tables and the Framingham Heart Study were used to calculate the effects of excess weight by class on loss of healthy life-years (defined as years without CVD or diabetes).15 Compared with normal weight, excess weight (grouped into overweight, class 1 obesity, and class 2 or 3 obesity categories) had a clear impact on the number of healthy life-years lost in both men and women, with greater losses seen among younger individuals (Figure 1).3,15 For example, among men and women aged 20 to 39 years, those with overweight lost approximately 6 more healthy life-years than did those with normal weight, whereas those with class 1 obesity lost approximately twice that, and those with class 2 or 3 obesity lost approximately 3 times that; this trend continued to a lesser degree among men and women aged 40 to 59 years and 60 to 79 years.15 In all age groups studied, excess body weight correlated with early development of CVD and T2D. Furthermore, the results showed relationships between BMI class and risk of hypertension; elevated serum glucose, triglyceride, and total and LDL cholesterol levels; and reduced HDL cholesterol levels.15
A retrospective analysis of cross-sectional data gathered from the 2018 National Health and Wellness Survey (NHWS) investigated correlations between adult obesity and comorbidities derived from a representative random sampling of 69,742 US adults; results showed that patients in higher obesity classes had higher rates of T2D. Rates of T2D were significantly higher among patients with class 3 obesity (25.3%) versus those with class 2 obesity (19.7%), individuals with class 2 obesity versus those with class 1 obesity (15.2%), people with class 1 obesity versus persons with overweight (9.1%), and those with overweight versus individuals of normal weight (3.3%; all comparisons, P < .05). The cross-sectional design of the NHWS, the use of bivariate statistics, and the reliance on BMI categories were limitations of this analysis.16
Cancer
Substantial associations have been established between higher BMI and increased risk of developing certain types of cancer.17 This pathophysiologic relationship may be explained partially by the association of obesity with a decrease in fat cell precursors maturing into adipocytes, which may indirectly result in increased levels of local and circulating proinflammatory cytokines and proangiogenic factors.18 A positive feedback loop then occurs, wherein the proinflammatory factors impair adipocyte maturation; this may result in conditions that favor tumor growth, both in the adipose microenvironment and, perhaps, at distant sites.18 Results of a meta-analysis showed that even a 5-kg/m2 increase in BMI was strongly associated with an increased risk of certain cancers, including thyroid and colon cancers in men, endometrial and gallbladder cancers in women, and esophageal adenocarcinoma and renal cancers in both sexes.19 The highest relative risk (RR) per 5-kg/m2 increase in BMI was shown for endometrial cancer in women (RR, 1.59; P < .0001) and for esophageal adenocarcinoma in men (RR, 1.52; P < .0001) and women (RR, 1.51; P < .0001). Another analysis of over 1000 epidemiologic studies found that when compared with individuals with a normal BMI, those with an elevated BMI had a greater risk of certain cancers of the gastric cardia, colon, rectum, liver, gallbladder, pancreas, kidney, breast (in postmenopausal women), uterus, ovary, and thyroid, as well as esophageal adenocarcinoma, meningioma, and multiple myeloma.17 In this analysis, comparison of the risk of cancer development among individuals with class 3 obesity versus those with normal weight showed that the RR was highest for endometrial cancer (RR, 7.1; 95% CI, 6.3-8.1) and esophageal adenocarcinoma (RR, 4.8; 95% CI, 3.0-7.7).17
Furthermore, the results of a 2017 report from the Centers for Disease Control and Prevention (CDC) indicated that the prevalence of overweight- and obesity-related cancers (excluding colorectal cancer) increased by 7% among US adults (age, ≥ 20 years) from 2004 to 2015, whereas the prevalence of cancers not known to be related to overweight or obesity decreased by 13%.20 Analyzed data compiled from the 2005-2014 US Cancer Statistics data set excluded colorectal cancer due to increased use of screening tests that likely contributed to its steep decline (–23%) over the period. The increase of adiposity-related cancer incidence mirrors the approximate 7% increase in obesity among US adults noted from 2003-2004 (32.2%) to 2015-2016 (39.6%).1
COVID-19
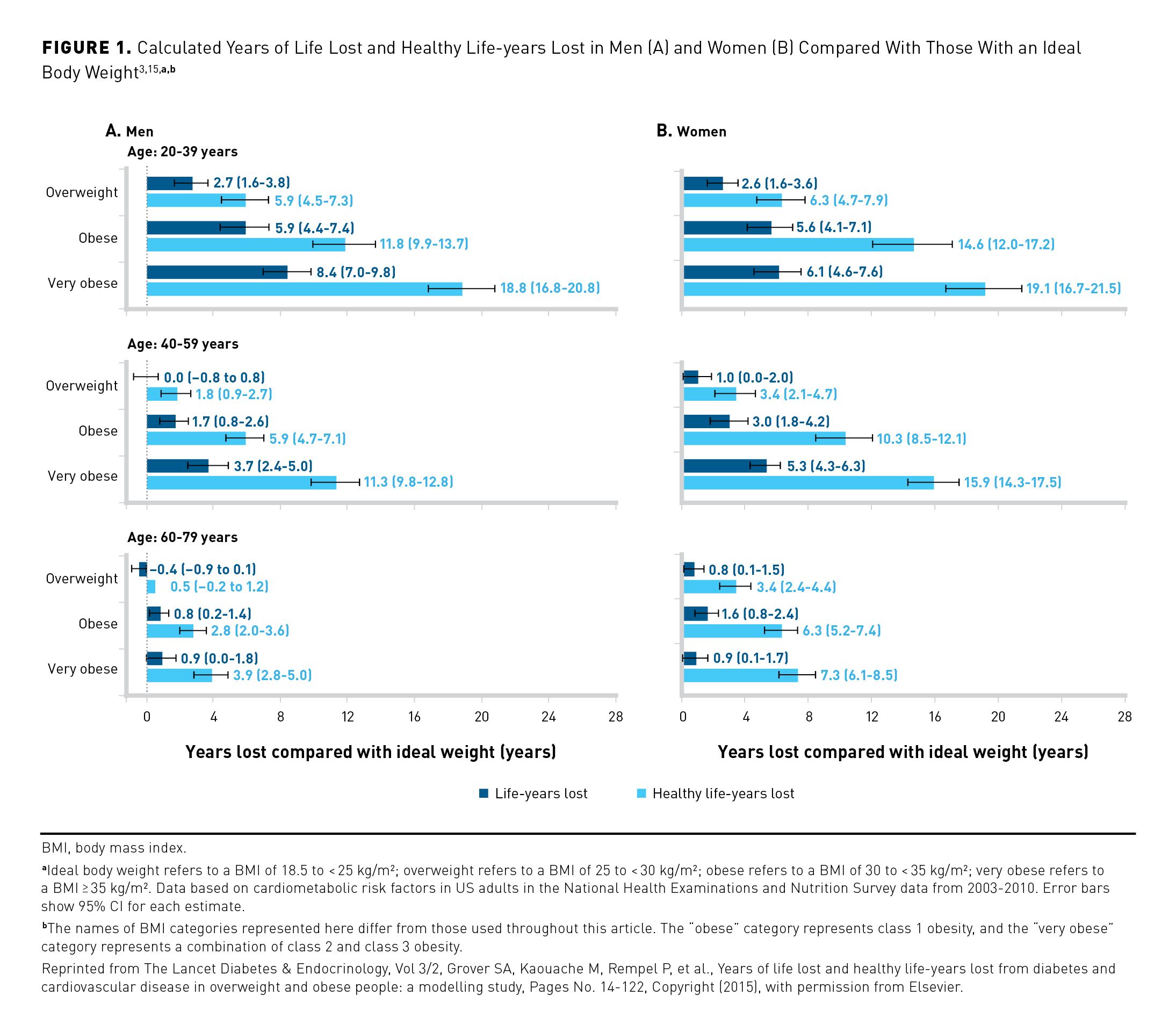
Obesity has emerged as a significant risk factor for morbidity and mortality among patients with COVID-19, with risk generally increasing in patients with higher BMIs. Investigators from the CDC analyzed the relationship between BMI and COVID-19 outcomes using data from the Premier Healthcare Database Special COVID-19 Release (PHD-SR). The data set included nearly 150,000 patients in US hospitals who were diagnosed with COVID-19 between March 2020 and December 2020. Obesity was found to be a risk factor for COVID-19 hospitalization, intensive care unit (ICU) admission, and invasive mechanical ventilation; furthermore, risk of hospitalization and death increased with increasing BMI class (Figure 2).21 These risk estimates were calculated entirely from adults receiving care at a hospital and may not be generalizable to all adults with COVID-19 or representative of the US patient population as a whole.21
A separate retrospective analysis of patients with COVID-19 seen in the Cleveland Clinic Health System between March 11, 2020, and July 30, 2020, reinforced trends seen in the previous CDC analysis relating to increased risk of hospitalization among those within the highest BMI classes. Among 2839 patients who recovered from acute infection and who did not require ICU admission, those with class 2 or class 3 obesity had a significantly higher risk for hospitalization (HR, 1.28 [95% CI, 1.05-1.56] and HR, 1.30 [95% CI, 1.06-1.59], respectively) compared to patients with normal BMI (P = .006), although admission rates of patients with overweight or class 1 obesity versus those with normal BMI were comparable. Notably, during a median follow-up time of 8 months through January 27, 2021, the analysis revealed that patients with obesity had an increased risk for post-acute sequelae of COVID-19 (PASC). The investigators could adjust outcomes by age, sex, race, and smoking status; however, adjustments according to preexisting medical conditions, laboratory values, and use of pharmacologic agents were precluded by a lack of information. Results of this study were further limited by the retrospective study design, use of electronic medical record (EMR) data from a single health system, and absence of records detailing why patients were hospitalized, underwent diagnostic testing, or died; these events, therefore, could have been unrelated to SARS-CoV-2 infection. Data on mild PASC that did not require diagnostic testing or hospitalization were not captured, nor were data on PASC in patients with asymptomatic COVID-19 who were not tested.22
Mortality
The relationship between obesity and mortality is multifaceted. Higher BMI correlates with an increased risk for certain leading causes of death (eg, cardiometabolic and cerebrovascular diseases, certain types of cancer) in the United States, and BMI greater than 40 kg/m2 can be a risk factor for premature death independent of comorbidities.3,13,17,23-25
This complexity was further demonstrated in a retrospective cohort study which used EMR data from 39,735 adult patients seen at the Mayo Clinic in Rochester, Minnesota, between January 1, 2000, and January 1, 2005 (median follow-up, 9.2 years). The residual mortality risk attributed to BMI showed a U-shaped distribution, in which individuals with very low BMI (< 15 kg/m2) or a very high BMI (≥ 45 kg/m2) had an elevated mortality risk not explained by comorbidities. Although baseline comorbidities were better predictors of mortality risk overall, the independent association between elevated BMI and mortality risk emerged clearly among patients with few or no comorbidities. The possibility of inaccurate or incomplete EMR data and other limitations generally associated with observational retrospective cohort studies should be considered when interpreting these results. Furthermore, data on patient smoking status were limited and nonstandard, so this variable was not considered. Generalizability of results may be limited due to data retrieved from a single medical center and the likely underrepresentation of healthy individuals.23
The association between BMI and mortality was further explored in the previously discussed modeling study using 2003-2010 NHANES data in non-Hispanic White US adults, which examined not only loss of healthy life-years but also average life expectancy losses due to overweight and obesity.15 For both men and women, the effect of excess weight on lost life-years was most pronounced among the younger population (those aged 20-39 years), underlining the cumulative effect of obesity on reducing lifespan (Figure 1).3,15
A separate retrospective cohort study examined the relationship between baseline BMI and mortality over more than 30 years of follow-up; results were based upon data from 273,843 patients who underwent health examinations at the Kaiser Foundation Health Plan of Northern California. Patients initially were seen between 1964-1973 or 1978-1985 and were followed through December 2012. In all, 103,218 deaths were recorded over the course of the study. Compared with patients with normal weight, the adjusted odds ratio (OR) for total mortality was 1.14 (95% CI, 1.11-1.17) for those with overweight, 1.49 (95% CI, 1.43-1.55) for those with class 1 obesity, 2.09 (95% CI, 1.93-2.26) for those with class 2 obesity, and 2.70 (95% CI, 2.40-3.03) for those with class 3 obesity (P < .001 for all). Among both men and women, mortality risk progressively increased for patients with class 2 and class 3 obesity when compared with individuals with normal weight. Because this study only used a single baseline measure of BMI and covariates, changes in weight either before or after baseline were not analyzed. Additionally, the study only captured deaths that occurred in California, and it did not examine diabetes, hypertension, or blood lipid measures. Data also were not controlled for certain relevant factors (eg, diet, exercise) that may impact mortality, and the absence of detailed adiposity-related data hindered efforts to account for sex and race disparities.26
The relationships between CVD-associated mortality and 5 factors that included obesity were analyzed in a study using mortality data from the 2000-2015 Surveillance, Epidemiology, and End Results data set; results based on 2012-2015 data revealed greater age-standardized mortality rates due to CVD premature death in US counties having higher rates of obesity.27 The results of another study showed that individuals in higher BMI categories had a greater risk of cardiovascular-related death than did those with normal weight.26 The OR for CVD death was 1.37 (P < .001) for those with overweight and 1.99 (P < .0001) for those with class 1 obesity. This trend continued among those with class 2 and class 3 obesity. Furthermore, results of The Atherosclerosis Risk in Communities study of 14,941 Black and White men and women (age, 45-64 years) showed that patients with greater BMI experienced a significantly increased risk of sudden cardiac death (P = .01).11 Thus, not only may obesity increase the risk of mortality due to CVD, but that risk may be higher among those with more severe obesity.
Current evidence also supports an association between obesity and increased mortality in patients with certain cancers. A meta-analysis of 203 studies from EMBASE, PubMed, and the Cochrane Library from database inception through September 2020 examined the association between obesity and cancer outcomes among adult patients with solid tumors overall and with specific cancer types.25 Compared to those with normal weight, patients with obesity had significant reductions in both overall survival (OS) (HR, 1.14 [95% CI, 1.09-1.19]) and cancer-specific survival (CSS) (HR, 1.17 [95% CI, 1.12-1.23]) (both P < .001). Significant reductions in OS related to breast, colorectal, and uterine cancers were noted among patients with obesity (breast: HR, 1.26 [95% CI, 1.2-1.33] and colorectal: HR, 1.22 [95% CI, 1.14-1.31] [both P < .001]; uterine: HR, 1.20 [95% CI, 1.04-1.38] [P = .01]). Similarly, CSS was decreased in patients with obesity and breast, colorectal, prostate, and pancreatic cancers (breast: HR, 1.23 [95% CI, 1.15-1.32] and colorectal: HR, 1.24 [95% CI, 1.16-1.32] [both P < .001]; prostate: HR, 1.26 [95% CI, 1.08-1.47] and pancreatic: HR, 1.28 [95% CI, 1.05-1.57] [both P = .01]). Although obesity was associated with increased mortality only in certain cancers, the overall trends support a link with increased cancer- and noncancer-related deaths. Limitations of the analysis include the heterogenous designs of studies analyzed and the inability to calculate an effect per BMI unit increase. Across studies, the use of patient self-reported height and weight measurements may have affected the accuracy of BMI data. In addition, data from patients with obesity were compared to the combined data of patients with normal and overweight. Finally, the authors noted that included studies rarely adjusted outcomes based on private insurance status. Obesity can increase costs for cancer treatment and lead to complications. Patients of lower socioeconomic status may have less access to medical facilities and treatments, rehabilitation, or follow-up intensity and, therefore, may experience worse health outcomes.25
Obesity also has been associated with an increased risk of morality among patients with COVID-19. The CDC study using PHD-SR data observed a positive correlation between BMI category and risk of COVID-19–related death. The adjusted RR for mortality ranged from 1.08 (95% CI, 1.02-1.14) in patients with class 1 obesity to 1.61 (95% CI, 1.47-1.76) in those with a BMI of 45 kg/m2 or greater. Notably, when considering only patients younger than 65 years with BMI of 45 kg/m2 or greater, the adjusted RR for COVID-19–related death rose to 2.01 (95% CI, 1.72-2.35), indicating the increased mortality risk experienced by younger adults with very high BMI.21
Quality of Life and Social Burdens of Obesity
Obesity’s association with poor outcomes extends beyond clinical diseases and conditions. Affected individuals may experience weight-related stigma and diminished physical, mental, and social quality of life (QOL) that can impact their well-being.16,28-30
The Awareness, Care, and Treatment in Obesity Management (ACTION) study included an online survey of 3008 US adults with class 1 obesity or higher. Investigators assessed health-related QOL (HRQOL) across obesity classes using 2 different measures. Both measures showed significantly lower HRQOL with increasing obesity class across all measured domains, including physical function, general health, mental health, sexual life, work, self-esteem, public distress, and social functioning (all differences between obesity classes, P < .05). Although patients can best share their own experiences related to these domains, the reliance on patient-reported outcomes and use of an online survey methodology may have limited the results of this analysis.29
Similarly, results from the previously discussed study of 2018 data from the NHWS assessed the relationship between BMI and QOL using patient-reported outcomes from 69,742 US adult survey respondents with normal or greater weight. Compared to respondents with normal weight, those with obesity reported lower HRQOL scores, with the largest reductions in HRQOL seen in mental and physical component scores for patients with class 2 and class 3 obesity. There were significantly lower scores across all domains for patients with class 2 versus class 1 obesity and for individuals with class 3 versus class 2 obesity; domains studied included physical and mental component scores, as well as individual scores for physical functioning, bodily pain, social functioning, mental health, vitality, general health, physical role limitations, and emotional role limitations (all P < .05).16
Stigma
Individuals with overweight and obesity face social stigmatization and discrimination, and they may be burdened by negative stereotypes about behavioral and moral characteristics based on the assumption of complete personal responsibility for excess weight. A consensus statement produced by a multidisciplinary international expert panel addressed the challenges of stigma faced by people with obesity in health care, workplace, and educational settings.30 Health care professionals may hold negative biases regarding obesity that can negatively affect the quality of health care provided to those with excess adiposity. Further, patients with obesity may be less likely to pursue or receive appropriate treatment for obesity or other conditions due to external or internalized weight-based stigma.30 The panel noted that women are more likely to experience weight-based discrimination than are men; furthermore, the consequences of stigma from both external sources and internalized beliefs and attitudes about obesity can combine to negatively impact an individual’s self-esteem and mental health and increase the individual’s susceptibility to depression, anxiety, stress, and substance abuse.30,31 Moreover, individuals in higher BMI classes may face greater prevalence of perceived weight discrimination.31 The panel also stated that those who experience this stigma are less likely to engage in physical activity and exercise and are more likely to have unhealthy diets and sedentary behaviors that may increase adiposity, creating a positive feedback loop and compounding the negative effects of weight-based discrimination.30
Economic Burden of Obesity
The high economic burden associated with obesity and related comorbidities represents an outsized proportion of health care costs in the United States. Direct comparisons between studies that seek to assess the direct and indirect costs of obesity are limited by methodological heterogeneity; nonetheless, these studies generally estimate a very large cost burden of excess adiposity and associated diseases to health care systems and society.6,32 The following summarizes data from recently published US-based studies.
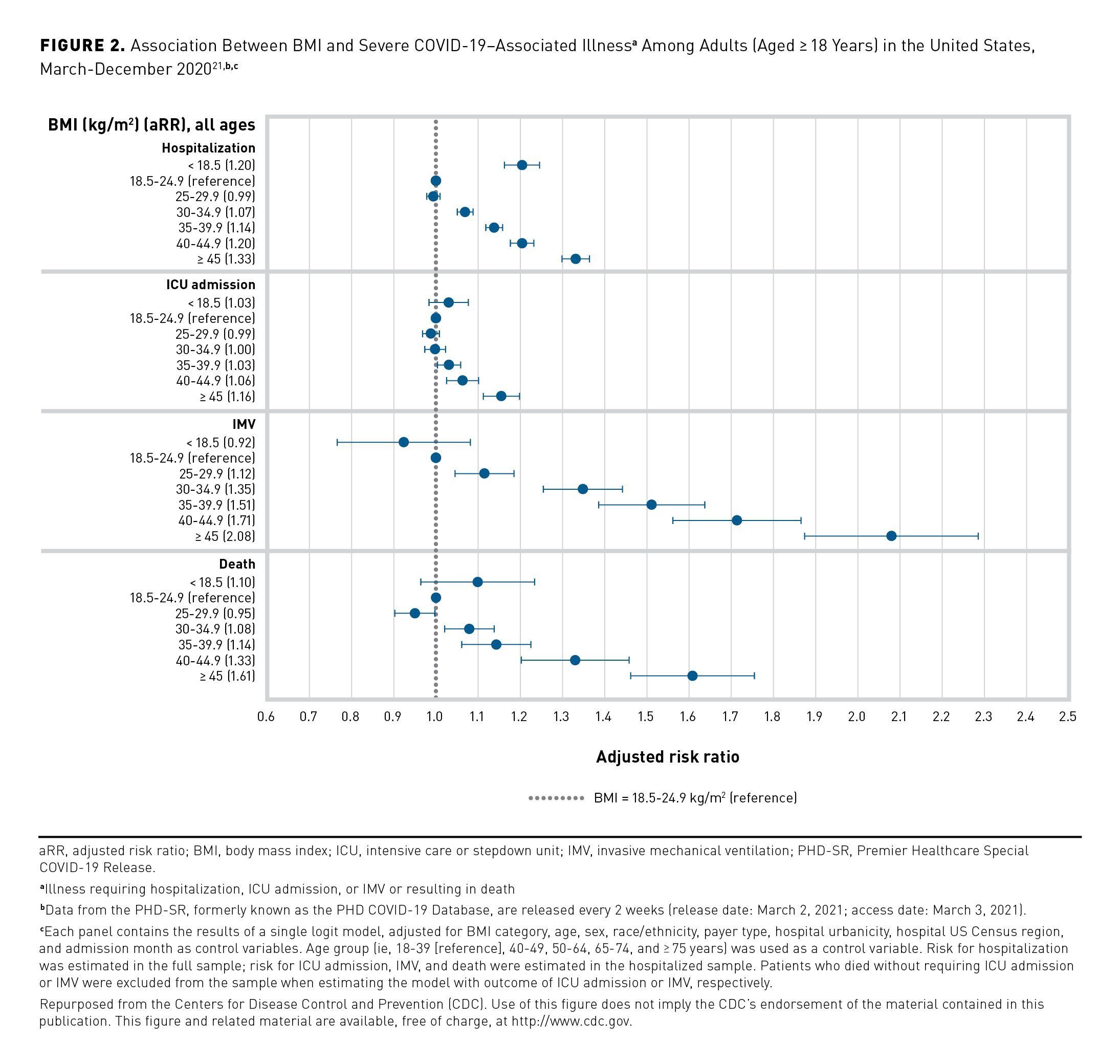
An analysis of medical literature on weight-related costs and data from the Medical Expenditure Panel Survey (MEPS) from the Milken Institute estimated that in 2016, chronic diseases associated with overweight and obesity caused $480.7 billion in direct medical costs and $1.24 trillion in indirect costs (2016 US$ for both) due to lost economic productivity, representing 47.1% of all direct and indirect chronic disease costs in the United States that year.6 The result was a total cost of $1.72 trillion, or approximately 9.3% of the US gross domestic product in 2016. A separate analysis of 2001-2015 MEPS data sought to determine the proportion of costs correlated with obesity to total US medical costs over time based on medical expenditures (2015 US$) from 334,297 adults, comparing excess expenditures among those with obesity (n = 99,377; 29.7% of sample) versus those of matched controls.33 A steady and significant increase in the proportion of national medical expenditures associated with people with obesity was observed (6.13% to 7.91%), representing a 29% increase over the 15-year period (P < .05) (Figure 3).33This followed the gradual increase in the prevalence of obesity seen in the United States over time (2001-2002, 30.5%; 2015-2016, 39.6%).1,33 On average, between 2010 and 2015, obesity expenditures accounted for 9.21%, 6.86%, and 8.48% of expenditures for private insurers, Medicare, and Medicaid, respectively.33 This study’s methodology allowed for a correlative, but not causal, determination of costs, although previous analyses indicated that correlative studies consistently underestimate the causal relationship between obesity and medical costs.
In a 2021 study, researchers investigated the causal effects of obesity on direct medical expenditures, employing a 2-part model to correct for both that segment of the population with zero expenditures and the positive-skewing of those with expenditures (2017 US$), since a small proportion of the latter group represents an outsized proportion of costs. The study, an analysis of a pooled cross-sectional sample of 2001-2016 MEPS data, only examined adult patients (N = 63,508) with at least 1 biological child to factor in the influence of inheritability of a propensity for weight gain in their analysis. Compared with those with normal weight, adults with obesity experienced a doubling in total annual medical care expenditures ($2504 vs $5010), with increases in expenditures of 68% for class 1 obesity ($1713), 120% for class 2 obesity ($3005), and 234% for class 3 obesity ($5850). The incremental cost per unit of BMI was $201 (90% CI, $149.37-$251.89). Persons with obesity experienced increased costs for every category of care—inpatient, outpatient, and prescription drugs—and higher obesity classes were significantly associated with greater expenditures in each category. The largest increases were seen for inpatient care, with class 1 obesity incurring 178% higher costs and class 3 obesity incurring 924% higher costs. Over the study period, total direct costs associated with obesity more than doubled from $124.2 billion in 2001 to $260.6 billion in 2016; in 2016, private health insurance paid $139.4 billion, public health insurance paid $57.9 billion, while patients paid $20.0 billion in out-of-pocket costs. The limitations of the study included possible errors associated with assumptions used in the 2-part cost model to predict the causal effect of BMI on medical expenditures; among these assumptions were that child BMI is not correlated with residual parental medical expenditures and that the effect of parental BMI on other outcomes does not affect child BMI. Both parts of the model were controlled for a number of variables (including race/ethnicity, gender and age of respondent and child, education level, location, employment and marital status, and health insurance coverage), but the authors acknowledged that BMI is a limited measure of adiposity and that child BMI may have been correlated with unknown factors for which the model could not account. Additionally, generalizability of results were limited by the inclusion criterion that patients have at least 1 biological child, which narrowed the age range of the study population.34
Projecting Future Direct Costs and Health Care Resource Utilization
A 2018 study employed 2000-2012 MEPS data to develop a model for projecting direct medical expenditures through 2025, with the MEPS data being reweighted to match a historical and a projected national population using data from NHANES and the US Census Bureau. The projection model sought to calculate health care use and expenditures (2010 US$) for different classes of obesity. Total health care expenditures are expected to more than double between 2000 and 2025; they are estimated to increase by 69% in patients with normal weight, 76% in those with class 2 obesity, and 93% in those with class 3 obesity. Use of all health care services except outpatient care is calculated to increase; the number of outpatient visits is projected to rise only among people with class 3 obesity. Patients with normal or overweight show stabilizing or even decreasing trends in use of inpatient and outpatient care, whereas use of nearly all health care services for those with class 2 and 3 obesity are expected to increase over time. Of note, MEPS data depend on self-reported information supplemented with administrative data for many of its results; this may have led to an underestimation of future health care expenditures.35
Indirect Costs
Obesity’s economic impact extends beyond direct medical costs. The considerable indirect economic burden of obesity on individuals, employers, and the government must be considered. A study employing a Markov-based microsimulation model that combined 2005-2012 NHANES data and 2008-2012 MEPS data determined the indirect US economic burden arising from obesity through a comparison with normal-weight controls matched by demographic and insurance type. Over a 10-year time horizon, the per-person estimated indirect burden of obesity included $6800 in lost income due to lack of labor force participation; $17,100 in lower earnings despite labor force participation; $2200 in lost productivity to employers due to work absenteeism; and an additional $1300 in Supplemental Security Income (SSI) payments received (2013 US$). Furthermore, indirect costs due to loss of personal income, lower earnings, and SSI payments increased with increasing BMI class. The model made assumptions using data from multiple sources due to the lack of a single longitudinal data source with a large enough population over time; in addition, confidence intervals could not be calculated, because certain data applied to the model either lacked standard error data or were inherently subjective.36
The results of a separate study indicated that absenteeism and productivity losses rose with increasing classes of obesity. Investigators employed a 2-part instrumental variables model (including only patients with a biological child in the household) that applied 2001-2016 MEPS data from 50,789 employed adults (age, 20-65 years) to estimate the dollar value of lost productivity due to job absenteeism. Workers with normal weight lost an average of 2.34 days per year; annual absenteeism was 2.07 days higher (88.5%) in people with class 1 obesity, 3.67 days higher (156.8%) in those with class 2 obesity, and 7.13 days (304.7%) higher in those with class 3 obesity, averaging 3.0 workdays of job absenteeism annually associated with obesity. Furthermore, each additional unit of BMI was associated with 0.24 extra days/year of missed work. Per-worker annual productivity losses also increased with BMI category, ranging from $186.65 to $373.31 for those with class 1 obesity, to $331.66 to $663.32 for individuals with class 2 obesity, and $643.27 to $1286.54 for those with class 3 obesity. On a national level, obesity may have accounted for productivity losses in the billions of dollars, with aggregate losses increasing over the study period. The study’s limitations related both to its use of a model for which validity cannot be proven and to its exclusion of those who did not have a biological child living at home, which narrowed the study population and possibly limited the generalizability of results. Further, the model could only account for employed individuals and did not consider presenteeism costs; in addition, BMI estimates were calculated from self-reported data, and authors acknowledged that BMI itself does not directly measure fat or body composition.37
Conclusions
The effects of obesity and weight-related comorbidities in the United States are considerable and multifaceted. Patients with overweight and obesity may experience a higher risk of morbidity and mortality, decreased QOL, and increased stigma and discrimination. Furthermore, obesity and related comorbidities are associated with substantial medical and indirect costs at the individual and national level, and these costs may rise with increasing BMI. The growing prevalence of overweight and obesity in the United States and the increase in health care resources that may be required to meet the needs of patients create an urgent problem. Providers, health care decision makers, and payers should consider this important population health issue and ensure the continuation or implementation of plans and programs to support diagnosis and appropriate treatment for those with excess adiposity.
Acknowledgements
This peer-reviewed supplement was funded by Novo Nordisk Inc. The authors acknowledge the professional medical writing support from Clinical Communications, a division of MJH Life Sciences®, Cranbury, NJ, which received funding support from Novo Nordisk Inc, Plainsboro, NJ. Novo Nordisk Inc. provided scientific and medical accuracy review of this publication.
Author Affiliations: Moda Health (DCM), Portland, Oregon; University of Pennsylvania (AA) Philadelphia, PA; Stony Brook University Hospital (MK), Stony Brook, NY.
Funding Source:This supplement was supported by Novo Nordisk.
Author Disclosures: Dr Amaro reports serving as a paid consultant or paid advisory board member for Novo Nordisk and has received grants from Altimmune and Eli Lilly. Dr Kaplan has received speaker honoraria from Novo Nordisk. Dr Massie reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (DCM, AA, MK); analysis and interpretation of data (AA); drafting of the manuscript (DCM, MK); and critical revision of the manuscript for important intellectual content (DCM, AA, MK).
Address Correspondence to: Danielle C. Massie, PharmD, Moda Health, 601 SW 2nd Ave, Portland, OR 97204. Email: danielle.massie@modahealth.com
References
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2017-2018. Centers for Disease Control and Prevention, National Center for Health Statistics. Reviewed February 8, 2021. Accessed October 7, 2022. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi:10.4158/EP161365.GL
- Defining adult overweight & obesity. Centers for Disease Control and Prevention. Reviewed June 3, 2022. Accessed October 7, 2022. https://www.cdc.gov/obesity/adult/defining.html
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25; suppl 2):S102-S138. doi:10.1161/01.cir.0000437739.71477.ee
- Waters H, Graf M. America’s obesity crisis: the health and economic costs of excess weight. Milken Institute. October 2018. Accessed October 7, 2022. https://milkeninstitute.org/sites/default/files/reports-pdf/Mi-Americas-Obesity-Crisis-WEB_2.pdf
- Yuen M, Earle R, Kadambi N et al. A systematic review and evaluation of current evidence reveals 195 obesity-associated disorders (OBAD). Poster presented at: ObesityWeek 2016; October 31-November 4, 2016; New Orleans, LA. Poster T-P-3166.
- Cornier MA. A review of current guidelines for the treatment of obesity. Am J Manag Care. 2022;28(suppl 15):S288-S296. doi:10.37765/ajmc.2022.89292
- Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(5):525-538. doi:10.1016/j.jacc.2019.11.044
- Powell-Wiley TM, Poirier P, Burke LE, et al; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984-e1010. doi:10.1161/CIR.0000000000000973
- Adabag S, Huxley RR, Lopez FL, et al. Obesity related risk of sudden cardiac death in the atherosclerosis risk in communities study. Heart. 2015;101(3):215-221. doi:10.1136/heartjnl-2014-306238
- Rahmani J, Haghighian Roudsari A, Bawadi H, et al. Relationship between body mass index, risk of venous thromboembolism and pulmonary embolism: a systematic review and dose-response meta-analysis of cohort studies among four million participants. Thromb Res. 2020;192:64-72. doi:10.1016/j.thromres.2020.05.014
- Cohen JB. Hypertension in obesity and the impact of weight loss. Curr Cardiol Rep. 2017;19(10):98. doi:10.1007/s11886-017-0912-4
- Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47-56. doi:10.2147/ITT.S73223
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3(2):114-122. doi:10.1016/S2213-8587(14)70229-3
- Rozjabek H, Fastenau J, LaPrade A, Sternbach N. Adult obesity and health-related quality of life, patient activation, work productivity, and weight loss behaviors in the United States. Diabetes Metab Syndr Obes. 2020;13:2049-2055. doi:10.2147/DMSO.S245486
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798. doi:10.1056/NEJMsr1606602
- Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45-57. doi:10.1146/annurev-med-121211-091527
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569-578. doi:10.1016/S0140-6736(08)60269-X
- Steele CB, Thomas CC, Henley SJ, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052-1058.
- Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death–United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):355-361. doi:10.15585/mmwr.mm7010e4
- Aminian A, Bena J, Pantalone KM, Burguera B. Association of obesity with postacute sequelae of COVID-19. Diabetes Obes Metab. 2021;23(9):2183-2188. doi:10.1111/dom.14454
- Li J, Simon G, Castro MR, Kumar V, Steinbach MS, Caraballo PJ. Association of BMI, comorbidities and all-cause mortality by using a baseline mortality risk model. PLoS One. 2021;16(7):e0253696. doi:10.1371/journal.pone.0253696
- Health, United States, 2019. Centers for Disease Control and Prevention; National Center for Health Statistics. 2021. Accessed October 7, 2022. doi:10.15620/cdc:100685
- Petrelli F, Cortellini A, Indini A, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213520.
doi:10.1001/jamanetworkopen.2021.3520 - Klatsky AL, Zhang J, Udaltsova N, Li Y, Tran HN. Body mass index and mortality in a very large cohort: is it really healthier to be overweight? Perm J. 2017;21(3):16-142. doi:10.7812/TPP/16-142
- Chen Y, Freedman ND, Albert PS, et al. Association of cardiovascular disease with premature mortality in the United States. JAMA Cardiol. 2019;4(12):1230-1238. doi:10.1001/jamacardio.2019.3891
- Taylor VH, Forhan M, Vigod SN, McIntyre RS, Morrison KM. The impact of obesity on quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27(2):139-146. doi:10.1016/j.beem.2013.04.004
- Kolotkin RL, Look M, Tomaszewski KJ, et al. Health-related quality of life in subgroups of a US-based, stratified sample of people with obesity. Poster presented at: Obesity Week 2017; October 29-November 2, 2017; Washington, DC. Accessed October 7, 2022. https://www.rethinkobesity.com/content/dam/
obesity/rethink-obesity/pdf-files/RESOURCES_ACTION_Study_Poster_OW_2017_USA.pdf - Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26(4):485-497. doi:10.1038/s41591-020-0803-x
- Spahlholz J, Baer N, König HH, Riedel-Heller SG, Luck-Sikorski C. Obesity and discrimination–a systematic review and meta-analysis of observational studies. Obes Rev. 2016;17(1):43-55. doi:10.1111/obr.12343
- Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14(4):435. doi:10.3390/ijerph14040435
- Biener A, Cawley J, Meyerhoefer C. The impact of obesity on medical care costs and labor market outcomes in the US. Clin Chem. 2018;64(1):108-117. doi:10.1373/clinchem.2017.272450
- Cawley J, Biener A, Meyerhoefer C, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27(3):354-366. doi:10.18553/jmcp.2021.20410
- Cecchini M. Use of healthcare services and expenditure in the US in 2025: the effect of obesity and morbid obesity. PLoS One. 2018;13(11):e0206703. doi:10.1371/journal.pone.0206703
- Su W, Huang J, Chen F, Mocarski M, Dall TM, Perrault L. Modeling the clinical and economic implications of obesity using microsimulation. J Med Econ. 2015;18(11):886-897. doi:10.3111/13696998.2015.1058805
- Cawley J, Biener A, Meyerhoefer C, et al. Job absenteeism costs of obesity in the United States: national and state-level estimates. J Occup Environ Med. 2021;63(7):565-573. doi:10.1097/JOM.0000000000002198

