- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Most Patients With Moderate to Severe Psoriasis Continue Secukinumab Treatment
More than 70% of patients continued treatment with secukinumab after the first year, a study finds.
Secukinumab (Cosentyx), a biologic therapy targeting IL-17A, demonstrated a high rate of persistence, with 72.7% of patients with moderate to severe psoriasis continuing treatment after 1 year.1
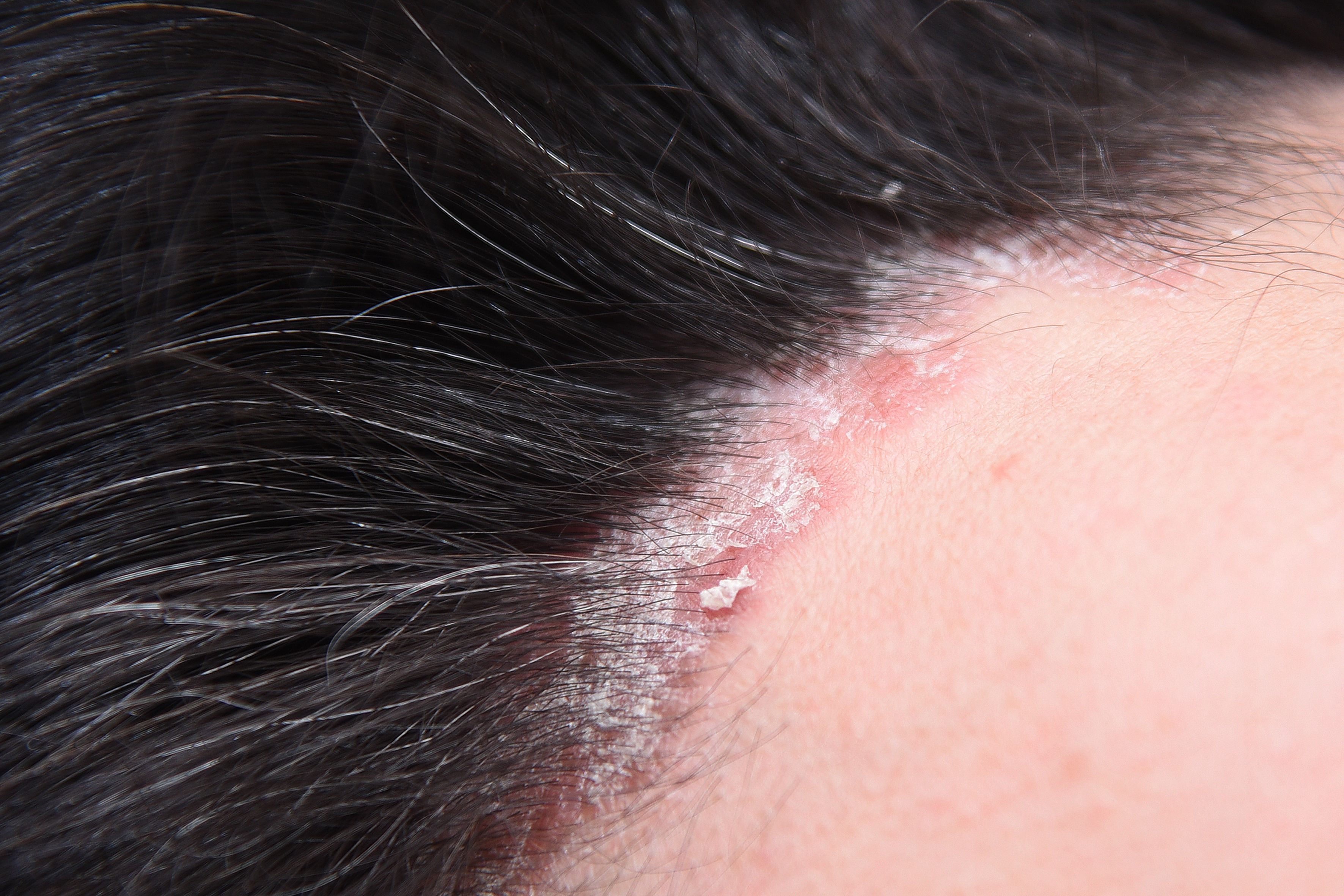
These results provide insights into the real-world performance of secukinumab, supporting its role in the long-term management of psoriasis.
The retrospective observational study is published in Farmacia Hospitalaria.
“Long-term control of moderate to severe psoriasis has been extremely important in the management of psoriasis, permitting the achievement of long-term remission of disease signs and symptoms in an increased proportion of patients,” wrote the researchers of the study. “Persistence is becoming a useful measure to evaluate the long-term effectiveness of therapies in real clinical setting in chronic diseases.”
Secukinumab is indicated for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.2 It is also indicated for active psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
For the indication of plaque psoriasis, the recommended dosage is 300 mg subcutaneous injection at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter. For some patients, a dose of 150 mg may be acceptable.
The study analyzed patients with moderate to severe psoriasis treated with secukinumab across 2 public hospitals in the region of Valencia, Spain, between February 2015 and March 2024.1 Eligible participants included adults who had failed glucocorticoid or immunosuppressant treatments and completed treatment with secukinumab. Data collected included demographics, clinical characteristics, prior treatments, Psoriasis Area Severity Index (PASI) scores, adverse events, and treatment discontinuation causes.
In addition to persistence and adherence calculations, health-related quality of life was measured using the Dermatology Life Quality Index (DLQI) across 6 domains of patient well-being.
The study included a total of 88 patients, 51.1% of whom were biologic nave. The mean (SD) baseline PASI score was 15.0 (2.9), and patients had previously received a mean (SD) of 1.4 (0.8) biologic treatments, most commonly anti–tumor necrosis factor-α (anti-TNFα) (60.5%) and ustekinumab (Stelara) (20.9%).
Secukinumab treatment was discontinued by 38.6% of patients, primarily due to lack of effectiveness (21.5%), partial response (9.2%), or adverse effects (7.9%). Overall, mean (SD) treatment persistence was 61.5 (21.7) months, with patients who had already taken a biologic showing slightly longer persistence (63.5 [12.4] months) compared with biologic-naive patients (54.1 [14.8] months; P = .804). The analysis also revealed persistence rates of 72.7%, 51.1%, and 39.8% at 1, 2, and 3 years, respectively, demonstrating substantial long-term retention among the cohort.
However, the researchers acknowledged some limitations to the study. First, the sample size was relatively small, which may have limited the generalizability of the results to a broader patient population. Second, the study was subject to potential biases, such as reliance on historical data and varying documentation quality.
Despite these limitations, the researchers believe the study adds real-world evidence showing secukinumab demonstrated long-term persistence in patients with moderate to severe psoriasis.
“The findings provide valuable insights into factors associated with treatment persistence, including prior exposure to biologic therapies,” wrote the researchers. “The article highlights the importance of long-term persistence data in evaluating the cost-effectiveness of biologic treatments for psoriasis.”
References
1. Borrás-Blasco J, Cornejo S, Valcuende-Rosique A, Alcala R, Bono AN. Long-term persistence with secukinumab in patients with moderate to severe psoriasis. Farm Hosp. Published online November 29, 2024. doi:10.1016/j.farma.2024.10.017
2. Secukinumab (Rx). Medscape. Accessed December 3, 2024. https://reference.medscape.com/drug/cosentyx-secukinumab-999964#0
Psoriasis as an Inflammatory Disease, and What’s Changed Over Time
August 3rd 2021August is National Psoriasis Awareness Month, and on this episode of Managed Care Cast, we bring you an excerpt of an interview with a New Jersey dermatologist about the changing concept of psoriasis as more than just a skin disease.
Listen
