- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Monoclonal Antibodies and JAK Inhibitors in Atopic Dermatitis Management: 2024 Guidelines and Managed Care Considerations
Introduction
ATOPIC DERMATITIS (AD), also known as atopic eczema, is a pruritic inflammatory skin disorder characterized by recurrent eczematous lesions with reddened, blistered, crusty, or oozing skin; lichenification; and xerosis.1-3 Its clinical presentation varies greatly, and signs and symptoms can manifest on any area of the body.1 The disease has a complex pathogenesis and heterogeneous presentation that is often associated with allergic, immune-mediated, and psychological comorbidities.1,4-6 Contrary to the common perception of it being primarily a childhood disease, AD affects approximately twice as many adults in the United States as it does children.7,8
The significant burden imposed by AD on patients and the health care system includes increases in hospitalizations, health care costs, loss of work productivity, and disability.9-14 This impact is particularly pronounced among patients with moderate or severe AD, because inadequate control of the disease can dramatically decrease quality of life (QOL).10,15
The 2024 American Academy of Dermatology (AAD) guidelines of care for the management of AD using phototherapy and systemic therapies in adults provide an important update to the AAD’s 2014 guidelines and offer recommendations on the use of monoclonal antibodies and JAK inhibitors.16 This article offers an overview of the clinical and economic impacts of AD, reviews the 2024 guidelines with a focus on strong recommendations, and discusses managed care considerations related to guideline implementation.
Clinical Impact
Pruritus, or itching, stands out as a central feature of AD and significantly contributes to the heavy burden experienced by patients.2,17 Outcomes of a study involving 380 adults with moderate to severe AD revealed that the majority reported experiencing daily itching, with nearly 60% describing it as unbearable or severe.10 In the United States, AD demonstrates a notable association with immune-mediated comorbidities such as ulcerative colitis and pernicious anemia.5 Moreover, adults with AD in the US exhibit a higher prevalence of cardiovascular conditions (eg, heart disease, high blood pressure) and psychological issues (eg, depression, anxiety) compared with those who are unaffected by the disease.6
The clinical impact of AD can be quantified through disability-adjusted life years (DALYs), a measure indicating the years of full health lost due to premature death or disability caused by a disease.18,19 Data from the Institute for Health Metrics and Evaluation’s Global Burden of Disease study indicated that in terms of all skin diseases in 2017, AD had the highest age-standardized DALY rate (123 per 100,000 individuals; 95% uncertainty interval [UI], 66.8-205 individuals) followed by psoriasis (70.0 per 100,000 individuals; 95% UI, 49.7-92.5 individuals).18
Further underscoring the burden of AD, an analysis of data from the 2013 US National Health and Wellness Survey (NHWS) revealed that adults with AD were more than twice as likely as matched controls without AD to report sleep disorders, depression, and anxiety (P < .001 for all).20 These statistics highlight the significant impact of AD on patients’ daily lives, mental well-being, and overall health.
Economic Impact
The economic impact of AD in the United States amounts to approximately $7 billion annually (2024 US$) for both direct and indirect costs.21-23 Direct costs include expenses related to visits to the hospital, emergency department (ED), and medical office and to prescription and over-the-counter medications.21 Indirect costs encompass expenditures associated with the time invested by patients, families, or caregivers in medical care; loss of work productivity; inability to engage in leisure activities; and costs of lost future earnings.21 In 2018, unadjusted annual health care costs for 31,164 adults with AD were $4979 higher than were costs for 93,492 matched controls ($14,603 vs $9624, respectively); this was primarily driven by increased outpatient services and pharmacy expenditures.24 Further analysis from health care claims data across various populations consistently shows that adults with AD have greater use of health care resources; further, higher disease severity correlates with increased ED visits, outpatient visits, and pharmacy prescriptions compared with not having a diagnosis of AD.25,26
2024 AAD Guidelines
The AAD’s 2024 guidelines for treating adults with AD using phototherapy and systemic therapies provide an update to the 2014 guidance. The treatment landscape has changed substantially since 2014. The 2024 update involves new evidence for established treatments and provides guidance for newer treatment options (eg, biologics, JAK inhibitors).16
The update was developed by an expert multidisciplinary work group using a systematic evidence review process. Members of the work group gathered safety and effectiveness data through systematic reviews and meta-analyses of randomized clinical trials (RCTs), where available, or consultations with experts in review groups. To ensure that patient values and preferences were considered, a patient representative was included, and a literature search for patient-reported outcomes, resource use, and feasibility was conducted. Assessment of evidence and formulation of recommendation strength used the Grading of Recommendations, Assessment, Development, and Evaluations framework.16
Recommendations discussed in the guidelines are for adults with moderate to severe disease. Concurrent use of topical agents with phototherapy or systemic agents is permitted for rescue, treatment of flares, or maintenance of response.16
The work group provided updated recommendations on therapies included in the 2014 guidelines. Conditional recommendations were made for the on-label use of phototherapy using UV radiation based on reports of the efficacy and safety of narrow-band UV-B. Conditional recommendations were also made for the off-label usage of methotrexate, mycophenolate, azathioprine, and cyclosporine with proper monitoring for adverse events (AEs). In response to immunosuppressants, members of the work group conditionally recommended against the use of systemic corticosteroids due to low-certainty evidence of efficacy and a substantial risk of serious AEs. Use of systemic corticosteroids should be limited to short courses in acute exacerbations for which no other options are available or as a bridge to other systemic therapy.16
Strong Recommendations
Strong recommendations were made for the use of monoclonal antibodies (biologics) and oral JAK inhibitors in adults with moderate to severe disease (Table 1).16
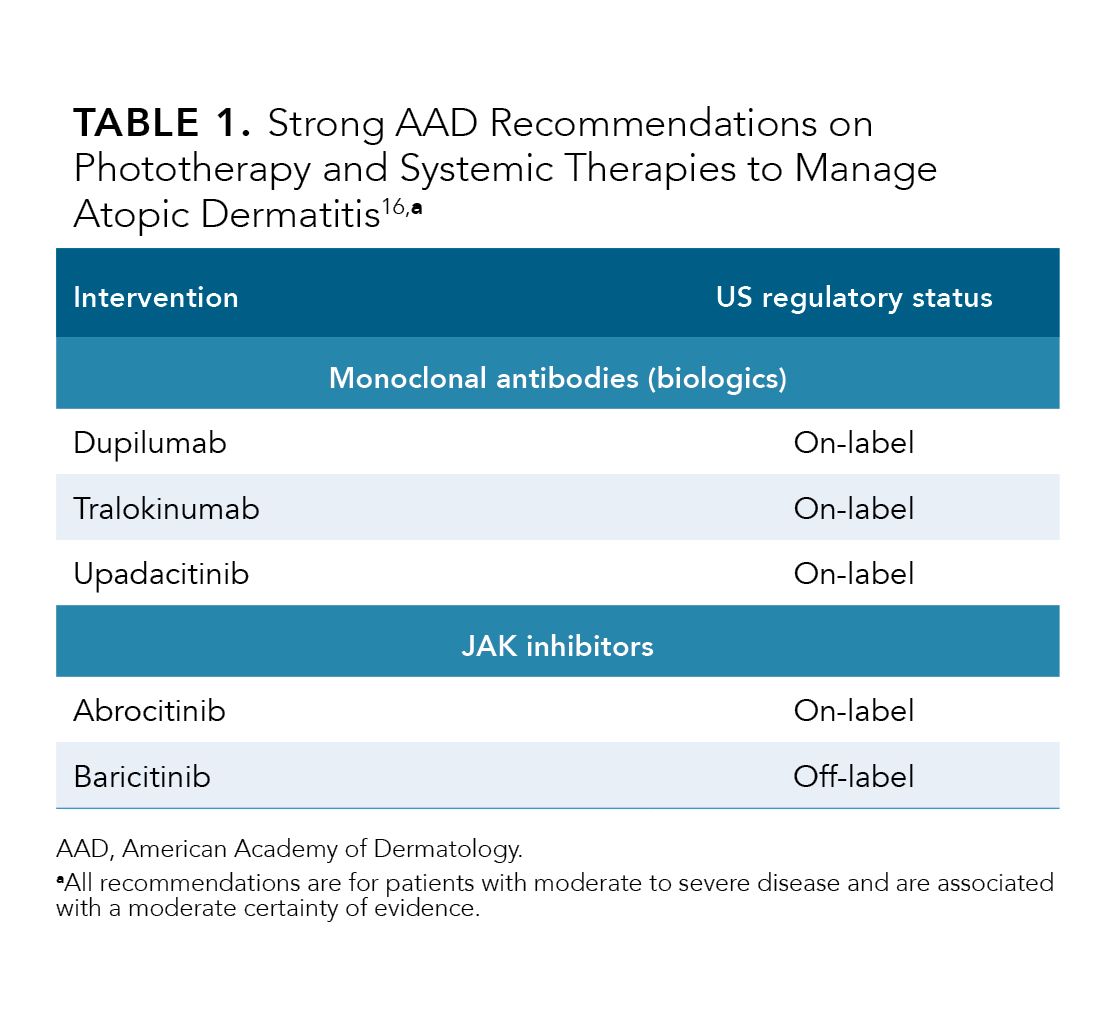
Monoclonal Antibodies
The updated AAD guidelines provided strong recommendations for the on-label use of dupilumab and tralokinumab.16 These biologics are FDA-approved for AD in adults; overall, their use has resulted in strong efficacy and safety data.16,27,28 However, due to statistical inconsistency in AE analyses, members of the work group downgraded the overall certainty of evidence for these agents to moderate.16 Unlike other systemic options for the treatment of AD, no laboratory monitoring is required with use of dupilumab and tralokinumab. Conjunctivitis remains a common AE with both monoclonal antibodies (incidence, ≥ 1%); however, it is generally self-limiting, and it can be managed with artificial tears.16,27,28
DUPILUMAB
The first FDA-approved targeted systemic treatment for AD was dupilimab, a human monoclonal antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling.27,29 Dupilumab is indicated for the treatment of adult and pediatric patients 6 months and older with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when use of those therapies is not advisable.27 The efficacy and safety of dupilumab in adults with moderate to severe AD was evaluated in 3 large RCTs (SOLO 1 [NCT02277743], SOLO 2 [NCT02277769], and LIBERTY AD CHRONOS [NCT02260986]) including a 52-week trial assessing the long-term outcomes and AEs.30,31 In 2024, an update of the dupilumab label included data that further supported use of the drug in patients with moderate to severe hand and foot AD involvement; this population is especially difficult to treat.32
More recent real-world evidence supports long-term use of dupilumab in AD. The PROSE trial (NCT03428646) is a prospective, observational, multicenter study conducted in the United States and Canada that is gathering real-world data from patients with AD who have received dupilumab therapy for at least 2 years.33 Patients 12 years or older with moderate to severe AD are given dupilumab per country-specific prescribing information. The collected data include objective clinician-assessed measures of AD disease severity (eg, body surface area [BSA] affected by AD, Eczema Area and Severity Index [EASI] scores) and patient-reported measures of symptoms and QOL (eg, Pruritus Numerical Rating Scale [P-NRS] and Dermatology Life Quality Index [DLQI]). Patients were assessed at baseline, at month 3, at month 6, and then every 6 months thereafter through year 5. Data are still being collected for this ongoing registry. At the time of data cut-off for this report, 632 of 764 enrolled patients remained in the study (mean duration of treatment, 18.9 months). In the interim analysis, consistent improvements in clinician-assessed measures and patient-reported outcomes were seen during the first clinic visit after 3 months of therapy; these effects were sustained throughout the 24-month observation period, supporting the long-term efficacy of dupilumab in AD. The drug was well tolerated in the cohort; AEs were consistent with experiences in previous clinical trials, with the most common being conjunctivitis (2.4%). Findings of this real-world evidence and claims-based analysis are limited by the lack of a comparator group, of control over concomitant adjunct treatments received by patients, and of the selection of sites and patients who chose to enroll in this study.33
Another real-world, retrospective cohort study was conducted in patients with AD to evaluate satisfaction with and tolerability of dupilumab therapy as shown by patient persistence with treatment at 12 months.34 Data from 2016-2018 were collected from IBM MarketScan commercial and Medicare claims databases, and patients 18 years or older were identified by 1 or more International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for AD. The index date was recorded as the first dupilumab dispensing event. Patients were assumed to have discontinued the medication if they did not have dupilumab dispensed again within the 30-day grace period between doses. Patients were considered reinitiators if they received dupilumab again after discontinuation. In all, 1963 patients were followed for an average of 314.5 days, and high rates of treatment persistence were observed. Persistence rates were 91.9% at 6 months and 77.3% at 12 months. Interestingly, 78.8% of individuals who discontinued therapy subsequently reinitiated treatment, usually within 4 months of discontinuation. These persistence rates were substantially higher than were real-world 12-month persistence rates found in other studies of patients with psoriasis who were given the biologics adalimumab (50%-62%) or infliximab (19%-50%).34
Patient-reported outcomes can provide valuable real-world insights into the impact of the disease on a patient’s QOL and daily functioning and may help improve management of AD.35 Patients can report on their mood, self-esteem, sleep disturbances, and ways that the condition affects their relationships and daily activities. In the prospective, longitudinal, real-world RELIEVE-AD study, patient-reported disease control and QOL were evaluated after the first year of dupilumab use for AD.35 Patients 18 years or older who had been prescribed dupilumab in routine clinical practice were enrolled in the trial and in the dupilumab patient support program. Patient outcomes were reported using the Atopic Dermatitis Control Tool (ADCT), a 6-point questionnaire evaluating symptom severity, number of days experiencing intense itch, and the degree to which AD was bothersome to affect sleep, daily activities, and mood over the past week. Additionally, patients reported the number of flares experienced over the past 4 weeks, documented their DLQI scores, and responded to the Work Productivity and Activity Impairment Questionnaire for AD (WPAI:AD) to elucidate a multifaceted evaluation of disease management. The survey was completed by 632 patients at month 1 and 483 patients at month 12. The results demonstrated rapid and sustained improvements in the patient-reported assessments throughout the study duration. The majority ( > 60%) of patients achieved adequate disease control (ADCT score, < 7) at month 1 with further improvement noted at each subsequent monthly follow-up; the mean (SD) ADCT score at month 12 was 4.4 (4.9). No flares were reported in 21 of 699 patients (3%) at baseline and 210 of 483 patients (43.5%) at month 12; no days of intense itch was reported in 22 of 699 patients (3.1%) at baseline and 257 of 483 patients (53.2%) at month 12. Substantial improvements in sleep behaviors were observed with the proportion of patients experiencing sleep problems decreasing from 77.5% (542 of 699 patients) to 14.1% (68 of 483 patients). Improvements in DLQI and WPAI:AD scores on all scales were also observed, and patient satisfaction was high (85.1%). The overall medication discontinuation rate was 10.9%, with AEs and lack of efficacy being the most common reasons for discontinuation.35
TRALOKINUMAB
In December 2021, tralokinumab became the second biologic indicated for the treatment of AD and the first treatment to specifically target IL-13.28,36 Tralokinumab is indicated for the treatment of moderate to severe AD in patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when use of those therapies is not advisable.28 The safety and efficacy of tralokinumab in treating adults with AD was evaluated in the ECZTRA series [ECZTRA 1 (NCT03131648), ECZTRA 2 (NCT03160885), and ECZTRA 3 (NCT03363854)] of 3 large, randomized, double-blind, placebo-controlled trials in subjects 18 years and older with a diagnosis of moderate to severe AD for at least 1 year that was not adequately controlled with topical therapies.37,38
Tralokinumab has not been evaluated in any head-to- head studies against other systemic therapies. In a systematic review and meta-analysis, tralokinumab showed slightly lower effectiveness compared with dupilumab after 16 weeks of treatment; use of tralokinumab resulted in a slightly reduced EASI score versus dupilumab (mean difference, –3.5; 95% CI, –5.8 to –1.3).39
Results from a 6-month interim analysis of an ongoing, 52-week, real world–evidence study evaluating patient-reported outcomes of AD treatment with tralokinumab were presented at the 2023 Fall Clinical Dermatology Conference.40 Data were collected from adult patients enrolled in the Adbry Advocate Program who completed surveys at baseline, at 1 month, and at 6 months; outcomes were stratified by previous dupilumab use. Of the 102 patients included in the interim analysis, half or more reported meaningful improvements in mean sleep, average weekly itch, and worst weekly itch NRS (57.1%, 57.1%, and 50.0%, respectively). Among all patients, 68.7% had an improvement in DLQI scores, with a 4.81-point improvement noted from baseline (range, 0-30 points). The study authors also identified a downward trend in the use of concomitant medications to treat AD. Treatment response varied within the cohort of patients who previously used dupilumab. Some individuals reported improvements in patient-reported outcomes, whereas others did not experience a response to treatment.40
Oral JAK Inhibitors
The updated AAD guidelines also provide strong recommendations for the use of systemic JAK inhibitors for the treatment of moderate to severe AD in adults including the on-label usage of upadacitinib and abrocitinib and the off-label usage of baricitinib.16 Upadacitinib and abrocitinib are FDA-approved for patients with moderate to severe AD for whom use of other systemic therapies, such as biologics, has failed to adequately manage disease or when use of other therapies are inadvisable.41,42 Upadacitinib and abrocitinib exhibit preferential targeting of JAK-1.41,42 Baricitinib, a dual JAK-1 and JAK-2 inhibitor, is approved for use in Europe in patients with moderate to severe AD.16,43 Its availability in the US for other immune-mediated conditions underscores its potential as an effective, off-label therapy for AD.16
The safety and efficacy of abrocitinib as monotherapy and in combination with topical corticosteroids (TCS) were established by the results of the 3 randomized, double-blind, placebo-controlled trials: JADE MONO-1 (NCT03349060), JADE MONO-2 (NCT03575871), and JADE COMPARE (NCT03720470).44-46 The safety and efficacy of upadacitinib were evaluated in 3 randomized, double-blind, multicenter trials—Measure Up 1 (NCT03569293), Measure Up 2 (NCT03607422), and AD Up (NCT03568318)— involving patients with moderate to severe AD who were not adequately controlled by topical medication and who were candidates for systemic therapy.47-49 Baricitinib is the first JAK inhibitor approved in the European Union and Japan to treat patients with moderate to severe AD who have an inadequate response to topical treatments.50 Despite not being approved by the FDA for this indication, baricitinib is widely used in clinical practice, and it has been given a strong recommendation by the updated AAD guidelines for use in adults with moderate to severe AD.16
All JAK inhibitors carry a black box warning regardless of treatment indication based on safety concerns from clinical trials investigating the JAK inhibitor tofacitinib in patients with rheumatoid arthritis.41,42,51,52 Serious AEs such as increased risk of serious heart-related events, cancer, blood clots, and death were seen in the tofacitinib studies.51
A systematic review and meta-analysis was conducted to determine the association of AD with incident venous thromboembolism (VTE) and to evaluate the risk of VTE among patients who were receiving treatment with JAK inhibitors for AD. The meta-analysis showed that compared with findings in cohorts receiving placebo or dupilumab, there was not an increased risk of VTE among patients with AD or a significant difference in the risk of incident VTE in patients with AD who were receiving JAK inhibitors. However, the relatively short durations of the included trials may limit these findings, and further data from long-term real-world evidence are needed.53
In clinical trials of patients with AD, AEs including death and thromboembolic events were also observed. Due to potential safety concerns with this class of medications, the JAK inhibitors are not regarded as first-line systemic therapy. To mitigate potential safety concerns, treatment should be initiated at lower doses, especially in older adults at greater risk.16
In addition to the cardiovascular risks, a potential for serious and opportunistic infections, including herpes zoster infection, is related to use of JAK inhibitors. Vaccination against shingles with special attention in older patient populations is recommended before JAK inhibitor therapy is begun. Furthermore, patients should receive necessary live vaccines before commencing treatment. The FDA advocates for comprehensive monitoring of patients on JAK inhibitors; this involves baseline assessments and subsequent evaluations. Recommendations include complete blood count with differential and liver enzyme checks at baseline along with additional monitoring after therapy initiation or dose escalation. Lipid assessments are advised after initiation; testing intervals vary for abrocitinib and upadacitinib. Baseline testing for viral hepatitis, tuberculosis, and pregnancy status is also recommended.16
The AAD’s strong recommendations for JAK inhibitors is supported by the high efficacy of these agents that has been demonstrated in clinical trials.16,44,46,49,54 As with the biologics dupilumab and tralokinumab, the overall certainty of evidence for JAK inhibitors was downgraded from high to moderate due to statistical inconsistency in AE analyses.16
Managed Care Considerations
The implementation of the updated AAD guidelines on systemic treatments for AD should involve a comprehensive approach that considers the limitations of long-term topical agents and Institute for Clinical and Economic Review (ICER) recommendations on the use of JAK inhibitors and monoclonal antibodies in AD treatment (Table 2).55

Shortcomings of Long-Term Use of Topical Agents
The 2024 AAD guidelines strongly recommend against the use of systemic corticosteroids.16 Moreover, medium- to high-potency TCS are not intended for daily, long-term use given the risk of systemic absorption and AEs.2 The guidelines permit the use of TCS and topical calcineurin inhibitors (TCIs) for maintenance of response, rescue, or treatment of flares as an adjunct to phototherapy or systemic therapy in patients with moderate to severe disease.16 However, findings from a study using 2015 data sourced from the Truven Health Analytics MarketScan Commercial and Medicare Supplemental databases showed that the use of TCS or TCIs for AD impacts pharmacy expenditures regardless of the treatment choice.56 Annual prescriptions per individual for TCS and/or TCIs ranged from 1 to 6. Similarly, the annual prescription cost per patient varied based on the type of medication (TCI vs TCS) and the strength of the TCS. While patients using medium-potency TCS alone had an annual prescription cost as low as $53.11, most patients had annual costs exceeding $250, with some reaching as high as $1465.03 each year.56
Recommendations From the 2021 ICER Report on JAK Inhibitors and Monoclonal Antibodies for the Treatment of AD
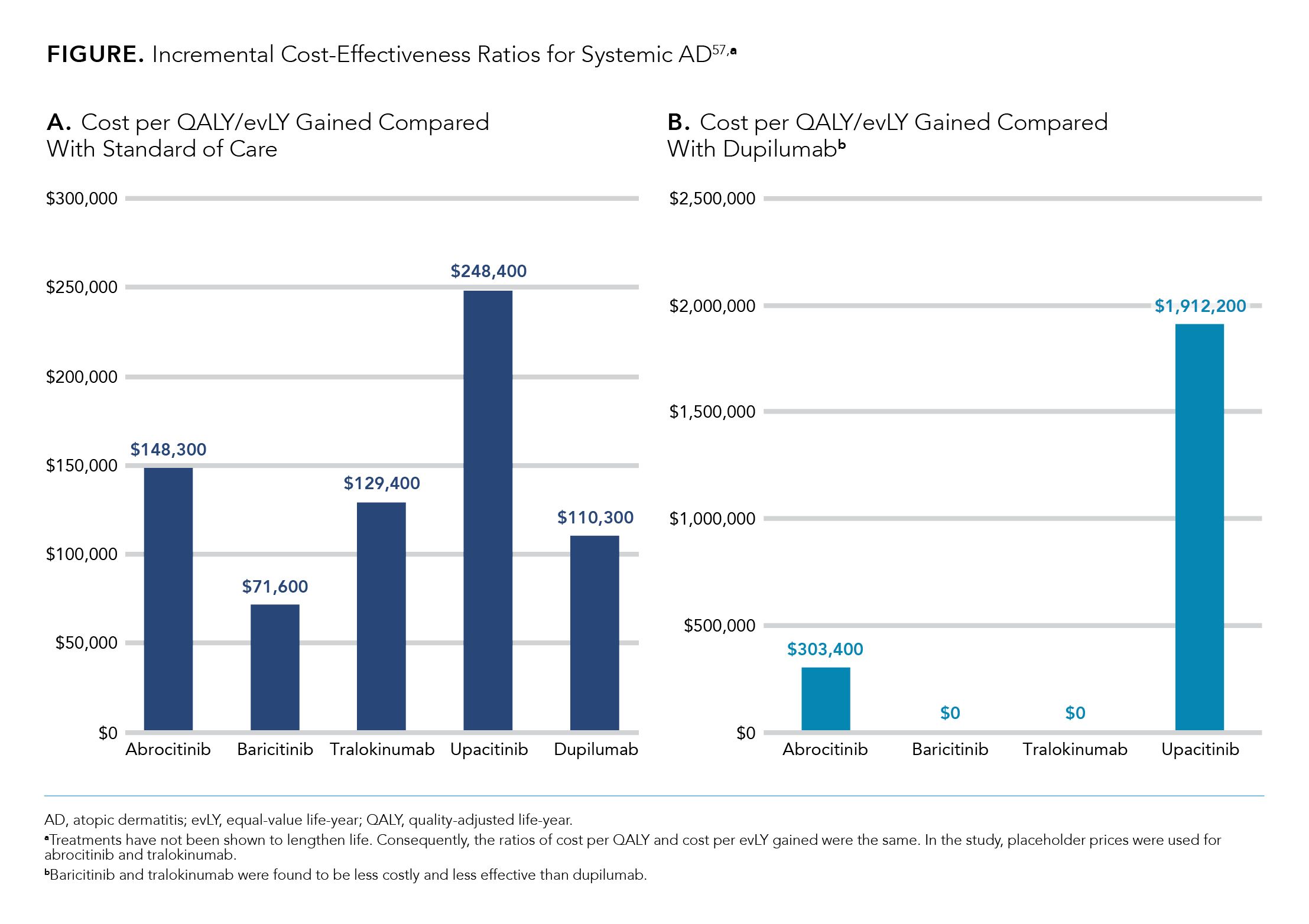
ICER is an independent nonprofit research institute dedicated to furthering evidence regarding the effectiveness and value of medications and various medical services.57 The institute’s reports feature evidence-based calculations of prices for new drugs that align with anticipated long-term improvements in patient outcomes.58 In 2021, ICER assessed the comparative clinical effectiveness and value of AD agents.57 The final evidence report (“JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: effectiveness and value”) compared tralokinumab, abrocitinib, baricitinib, and upadacitinib with topical emollients and dupilumab and offered policy recommendations for stakeholders.57
Using a Bayesian network meta-analysis based on available data from monotherapy RCTs, the authors conducted a quantitative, indirect comparison of the systemic treatments for AD. The authors concluded that the net health benefit of baricitinib and tralokinumab were comparable or inferior when compared with dupilumab and that the evidence for net health benefit for abrocitinib and upadacitinib compared with dupilumab was insufficient. The authors also favored dupilumab with respect to safety, citing both the agent’s long-term safety profile and concerns surrounding the safety of oral JAK inhibitors. Finally, the authors cited dupilumab’s proven efficacy in patients with comorbid conditions such as asthma or chronic rhinosinusitis.57
The authors also conducted a cost-effectiveness analysis comparing the oral JAK inhibitors with dupilumab and using placeholder prices for abrocitinib and tralokinumab. This demonstrated that baricitinib and tralokinumab would be less costly and less effective than dupilumab (Figure A).57 As compared with dupilumab, the cost per QALY or equal-value life year gained with use of abrocitinib and upadacitinib would be $303,400 and $1,912,200, respectively (2021 US$) (Figure B).57
Ensuring Access to at Least 1 Monoclonal Antibody and 1 JAK Inhibitor
When considering formulary coverage for severe AD, the ICER report recommends that if multiple agents for severe AD are approved, payers include at least 1 biologic and at least 1 JAK inhibitor. This approach recognizes the distinct characteristics of these medication classes including differences in onset of action and risk profiles. The offer of both classes of medications gives patients and health care providers access to diverse treatment options that cater to individual preferences, responses, and safety profiles.57
Accounting for Multiple Measures of Disease Severity
The ICER report advises payers to develop a broad, clinically relevant definition of AD that includes multiple specific measures of disease intensity. This recommendation accounts for the lack of a universally accepted definition of moderate to severe disease. It also reflects clinicians’ lack of familiarity with AD scoring systems used in clinical trials. Finally, the recommendation acknowledges that severity of AD can fluctuate significantly over time and that it encompasses a multitude of factors from the patient’s perspective (eg, itch intensity, affected body areas, skin impairment, and overall QOL impact).57 The report authors noted that a definition of moderate to severe AD might include, for instance, “any of the following: Body Surface Area (BSA) ≥ 10%, Investigator’s Global Assessment (IGA) ≥ 3, Eczema Area and Severity Index (EASI) ≥ 16,” or “affected BSA ≥ 10% OR involvement of challenging-to-treat body sites (e.g., hands, feet, face, neck, scalp, genitals/groin, skin folds) or severe itch unresponsive to topical therapies.”57 Whereas some payers may accept clinician attestation, others may base criteria on clinical trial eligibility.57 Clinical eligibility criteria are crucial in determining access to newer-generation treatments for AD, especially considering the diverse range of patients with varying levels of severity and the potential for AEs associated with these medications. However, it is equally important that limitations imposed by payers do not unduly restrict patient access to necessary therapies.
Limitations to Step Therapy
ICER report authors also advise that formulary decision makers approach step therapy for AD with flexibility, transparency, and efficiency to meet the diverse needs of patients.57
Clinical experts and patient representatives note common challenges such as delayed access to treatment, documentation burdens, and inadequate procedures for exceptions that lead to frustration and poor outcomes for some patients. Payers should consider offering at least 1 biologic and 1 oral JAK inhibitor as first-step therapy options, recognizing the differences in onset of action and risk profiles between these classes of drugs. The heterogeneity of AD supports the need for access to a range of different therapies rather than limiting step therapy to only 1 treatment option across biologics and oral JAK inhibitors. Step therapy policies should consider patient-specific characteristics and offer options that align with their particular needs (eg, periodic use of a JAK inhibitor for patients with seasonal disease, biologic treatment for patients with more year-round severity or asthma). Overall, the guidance underscores the importance of considering individual patient needs, clinical appropriateness, and treatment efficacy when implementing step therapy for AD.57
Conclusions
Commonly known as eczema, AD is a prevalent, chronic skin condition characterized by itchy, inflamed, and dry lesions that can persist or recur. It affects both children and adults, impacting various aspects of their lives including sleep, psychological well-being, and productivity. Further, this disease is associated with substantial clinical and economic impact. There are several treatment options that have shaped the landscape of systemic therapy for AD management; these include the biologics dupilumab and tralokinumab and the oral JAK inhibitors abrocitinib, baricitinib, and upadacitinib.
The AAD published updated clinical guidelines in 2024 for the use of phototherapy and systemic therapies to treat adults with AD; these guidelines strongly recommend that monoclonal antibodies and JAK inhibitors be given as systemic treatments for moderate to severe disease. These guidelines build upon the previous 2014 recommendations for AD management in patients who do not respond to topical therapies and underscore the importance of incorporating multiple measures of disease severity. Implementation of these guidelines is crucial to ensure equitable access to effective AD treatments. To promote effective new treatment options that reduce health inequalities, health care providers should incorporate findings from the 2021 ICER review on the clinical and cost-effectiveness of treatments for AD as they manage this chronic disease.
REFERENCES
1. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi:10.1038/s41572-018-0001-z
2. Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10-22.e2. doi:10.1016/j.anai.2017.10.039
3. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351. doi:10.1016/j.jaad.2013.10.010
4. Fishbein AB, Silverberg JI, Wilson EJ, Ong PY. Update on atopic dermatitis: diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract. 2020;8(1):91-101. doi:10.1016/j.jaip.2019.06.044
5. Narla S, Silverberg JI. Association between atopic dermatitis and autoimmune disorders in US adults and children: a cross-sectional study. J Am Acad Dermatol. 2019;80(2):382-389. doi:10.1016/j.jaad.2018.09.025
6. Silverberg JI, Gelfand JM, Margolis DJ, et al. Association of atopic dermatitis with allergic, autoimmune, and cardiovascular comorbidities in US adults. Ann Allergy Asthma Immunol. 2018;121(5):604-612.e3. doi:10.1016/j.anai.2018.07.042
7. Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283-289. doi:10.1016/j.det.2017.02.002
8. Table: estimates of the total resident population and resident population age 18 years and older for the United States, States, and Puerto Rico: July 1, 2023. Hawaii.gov. Accessed May 24, 2024. https://files.hawaii.gov/dbedt/census/popestimate/2023/state-pop/SCPRC-EST2023-18+POP.pdf
9. Whiteley J, Emir B, Seitzman R, Makinson G. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin. 2016;32(10):1645-1651. doi:10.1080/03007995.2016.1195733
10. Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491-498. doi:10.1016/j.jaad.2015.10.043
11. Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26-30. doi:10.1016/j.jid.2016.07.012
12. Atopic dermatitis. National Eczema Association. Accessed April 26, 2024. https://nationaleczema.org/eczema/types-of-eczema/atopic-dermatitis/
13. Silverberg JI. Associations between atopic dermatitis and other disorders. F1000Res. 2018;7:303. doi:10.12688/f1000research.12975.1
14. Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583-590. doi:10.1016/j.jid.2018.08.028
15. Simpson EL, Guttman-Yassky E, Margolis DJ, et al. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol. 2018;154(8):903-912. doi:10.1001/jamadermatol.2018.1572
16. Davis DMR, Drucker AM, Alikhan A, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90(2):e43-e56. doi:10.1016/j.jaad.2023.08.102
17. Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57. doi:10.1016/j.jaci.2017.01.009
18. Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. 2021;184(2):304-309. doi:10.1111/bjd.19580
19. Murray CJL, Lopez AD, World Health Organization, World Bank & Harvard School of Public Health. The Global Burden of Disease : A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020: Summary. Murray CJL, Lopez AD, eds. World Health Organization; 1996.
20. Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274-279.e3. doi:10.1016/j.jaad.2017.04.019
21. Bickers DR, Lim HW, Margolis D, et al; American Academy of Dermatology Association; Society for Investigative Dermatology. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490-500. doi:10.1016/j.jaad.2006.05.048
22. Adamson AS. The economics burden of atopic dermatitis. Adv Exp Med Biol. 2017;1027:79-92. doi:10.1007/978-3-319-64804-0_8
23. Consumer Price Index (CPI) inflation calculator. US Bureau of Labor Statistics. September 2023. Accessed April 26, 2024. https://data.bls.gov/cgi-bin/cpicalc.pl
24. Manjelievskaia J, Boytsov N, Brouillette MA, Onyekwere U, Pierce E, Goldblum O, Bonafede M. The direct and indirect costs of adult atopic dermatitis. J Manag Care Spec Pharm. 2021;27(10):1416-1425. doi:10.18553/jmcp.2021.27.10.1416
25. Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the commercial, Medicare, and Medi-Cal databases. Adv Ther. 2017;34(8):1989-2006. doi:10.1007/s12325-017-0582-z
26. Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78(1):54-61.e1. doi:10.1016/j.jaad.2017.08.002
27. Dupixent. Prescribing information. Regeneron Pharmaceuticals, Inc; 2024. Accessed May 1, 2024. https://www.regeneron.com/downloads/dupixent_fpi.pdf
28. Adbry. Prescribing information. LEO Pharma Inc; 2023. Accessed May 1, 2024. https://mc-df05ef79-e68e-4c65-8ea2-953494-cdn-endpoint.azureedge.net/-/media/corporatecommunications/us/therapeutic-expertise/our-product/adbrypi.pdf?rev=da521f7b67044a209c6876440c38400d
29. FDA approves new eczema drug Dupixent. Press release. FDA. March 28, 2018. Accessed April 26, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-eczema-drug-dupixent
30. Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med.;375(24):2335-2348. doi:10.1056/NEJMoa1610020
31. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287-2303. doi:10.1016/S0140-6736(17)31191-1
32. Media update: Dupixent (dupilumab) U.S. label updated with data further supporting use in atopic dermatitis with moderate-to-severe hand and foot involvement. Press release. Sanofi. January 16, 2024. Accessed April 25, 2024. https://www.sanofi.com/en/media-room/press-releases/2024/2024-01-16-12-00-00-2809681
33. Simpson EL, Lockshin B, Lee LW, Chen Z, Daoud M, Korotzer A. Real-world effectiveness of dupilumab in adult and adolescent patients with atopic dermatitis: 2-year interim data from the PROSE registry. Dermatol Ther (Heidelb). 2024;14:261-270. doi:10.1007/s13555-023-01061-4
34. Silverberg JI, Guttman-Yassky E, Gadkari A, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):40-45. doi:10.1016/j.anai.2020.07.026
35. Strober B, Mallya UG, Yang M, et al. Treatment outcomes associated with dupilumab use in patients with atopic dermatitis: 1-year results from the RELIEVE-AD study. JAMA Dermatol. 2022;158(2):142-150. doi:10.1001/jamadermatol.2021.4778
36. LEO Pharma announces FDA approval of Adbry (tralokinumab-ldrm) as the first and only treatment specifically targeting IL-13 for adults with moderate-to-severe atopic dermatitis. Press release. National Eczema Association. Updated December 28, 2021. Accessed April 25, 2024. https://nationaleczema.org/blog/leo-122821/
37. Wollenberg A, Blauvelt A, Guttman-Yassky E, et al; ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021 Mar;184(3):437-449. doi:10.1111/bjd.19574
38. Silverberg JI, Adam DN, Zirwas M, et al. Tralokinumab plus topical corticosteroids as needed provides progressive and sustained efficacy in adults with moderate-to-severe atopic dermatitis over a 32-week period: an ECZTRA 3 post hoc analysis. Am J Clin Dermatol. 2022;23(4):547-559. doi:10.1007/s40257-022-00702-2
39. Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523-532. doi:10.1001/jamadermatol.2022.0455
40. Lio P, Kim Y, Balu S, Costantino, H, et al. Tralokinumab real-world patient-reported outcomes in moderate-to-severe atopic dermatitis adult patients in the United States: 6-month interim analysis. SKIN J Cutan Med. 2024;8(1):s318. doi:10.25251/skin.8.supp.318
41. Rinvoq. Prescribing information. AbbVie Inc; 2024. Accessed May 1, 2024. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf
42. Cibinqo. Prescribing information. Pfizer Inc; 2023. Accessed May 1, 2024. https://labeling.pfizer.com/ShowLabeling.aspx?id=16652
43. Annex I. Summary of product characteristics. Olumiant (baricitinib). European Medicines Agency. Accessed April 25, 2024. https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf
44. Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255-266. doi:10.1016/S0140-6736(20)30732-7
45. Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863-873. doi:10.1001/jamadermatol.2020.1406
46. Bieber T, Simpson EL, Silverberg JI, et al; JADE COMPARE Investigators. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101-1112. doi:10.1056/NEJMoa2019380
47. Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404-413. doi:10.1001/jamadermatol.2022.0029
48. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168. doi:10.1016/S0140-6736(21)00588-2
49. Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate- to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169-2181. doi:10.1016/S0140-6736(21)005894
50. Updates on Olumiant (baricitinib) phase 3 lupus program and FDA review for atopic dermatitis. Press release. Eli Lilly and Company. January 28, 2022. Accessed March 8, 2024. https://investor.lilly.com/news-releases/news-release-details/updates-olumiantr-baricitinib-phase-3-lupus-program-and-fda
51. Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13(3):729-749. doi:10.1007/s13555-023-00892-5
52. Olumiant. Prescribing information. Eli Lilly and Company; 2022. Accessed May 1, 2024. https://uspl.lilly.com/olumiant/olumiant.html#pi
53. Chen TL, Lee LL, Huang HK, Chen LY, Loh CH, Chi CC. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(11):1254-1261. doi:10.1001/jamadermatol.2022.3516
54. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047-1055. doi:10.1001/jamadermatol.2021.3023
55. Agboola F, Atlas SJ, Brouwer E, et al. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: effectiveness and value. J Manag Care Spec Pharm. 2022;28(1):108-114. doi:10.18553/jmcp.2022.28.1.108
56. Clark R, Bozkaya D, Levenberg M, Faulkner S, Smith TW, Gerber RA. Topical treatment utilization for patients with atopic dermatitis in the United States, and budget impact analysis of crisaborole ointment, 2. J Med Econ. 2018;21(8):770-777. doi:10.1080/13696998.2018.1470520
57. Atlas SJ, Brouwer E, Fox G, et al. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: effectiveness and value; evidence report. Institute for Clinical and Economic Review. August 17, 2021. Accessed May 2, 2024. https://icer.org/wp-content/uploads/2020/12/Atopic-Dermatitis_Final-Evidence-Report_081721.pdf
58. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review. Updated February 27, 2023. Accessed April 25, 2024. https://icer.org/wp-content/uploads/2023/02/Atopic-Dermatitis_Final-Evidence-Report_Unmasked_02272023.pdf
For other articles and videos in this AJMC® Perspectives publication, please visit "Managing Atopic Dermatitis: Clinical Considerations, Payer Perspective, and 2024 Guidelines"
