
- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Medication-Related Problems Identified by Pharmacists in an Enhanced Medication Therapy Management Model
Objectives: To describe the types of clinically actionable medication-related problems (MRPs) identified and the types of resolving recommendations issued by pharmacists using an advanced clinical decision support system (CDSS) for Medicare Part D beneficiaries.
Study Design: Retrospective cross-sectional study.
Methods: We examined frequencies of MRPs and recommendations for beneficiaries who received a first-ever medication safety review (MSR) during plan years 2018-2019. MRPs were considered clinically actionable if implementation of a recommendation would alter the medication regimen.
Results: Pharmacists identified ≥ 1 clinically actionable MRP for 82.4% (18,703/22,696) beneficiaries receiving an MSR. Among these beneficiaries, 36,455 MRPs were identified (mean [SD] number of MRPs: 1.9 [1.0]). “Adverse drug reaction” (n = 14,788; 40.6%), “drug interaction” (n = 9716; 26.7%), and “medication use without indication” (n = 6496; 17.8%) represented 85.0% of all MRPs. “Start alternative therapy” was most frequently recommended to resolve “adverse drug reactions” (6724/14,788; 45.5%), followed by “change time of administration” to resolve “drug interactions” (5189/9716; 53.4%) and “discontinue medication” to resolve “drug use without indication” (5718/6496; 88.0%). Overall, “start alternative therapy” (n = 12,219) and “discontinue medication” (n = 12,059) made up 66.6% of all recommendations.
Conclusions: In Medicare Part D beneficiaries, pharmacists using an advanced CDSS identified a substantial number of MRPs pertaining to medication safety and issued recommendations to decrease the risk of adverse drug events.
Am J Manag Care. 2021;27(suppl 16):S292-S299. https://doi.org/10.37765/ajmc.2021.88754
For author information and disclosures, see end of text.
Introduction
In 2003, the Medicare Prescription Drug, Improvement, and Modernization Act established a prescription drug benefit (ie, Part D) that required Prescription Drug Plans (PDPs) to offer medication therapy management (MTM) services as a way to optimize beneficiary medication use.1 Whereas some PDPs have realized improvements in patient outcomes such as adherence and hospitalization costs,2 MTM has been characterized by poor beneficiary enrollment and substantial performance variation between PDPs in both drug therapy outcomes (eg, resolution of drug interactions, avoidance of contraindicated medications) and health care resource utilization (eg, Medicare costs, hospitalization rates, emergency department visits).3,4 In 2017, the CMS—through the Center for Medicare & Medicaid Innovation (CMMI)—launched a 5-year “Enhanced MTM” model that is testing whether providing PDPs with regulatory flexibility and financial incentives can promote enhancements to the MTM program, such as improved therapeutic outcomes and reduced net Medicare expenditures. For participating PDPs, the model offers an opportunity to identify and implement innovative strategies that optimize medication use.5
For Blue Cross and Blue Shield Northern Plains Alliance (BCBS-NPA) —a PDP participating in CMMI’s model—adopting the MedWise Risk Score (MRS)6,7 represents a new paradigm for targeting beneficiaries. Unlike traditional MTM programs that target beneficiaries based upon the number of chronic diseases, number of drugs, and Part D costs,3 the MRS identifies beneficiaries who have modifiable risk factors related to their medication regimens. This new approach uses beneficiaries’ drug-related claims data to determine their medication regimen; then, pharmacokinetic and pharmacodynamic characteristics of identified active ingredients feed algorithms that compute the MRS. The previous article8 in this supplement found that a higher MRS was associated with a plethora of poor outcomes among BCBS-NPA’s beneficiaries: increased odds of adverse drug events (ADEs), higher Parts A and B costs, more hospitalizations, more emergency department visits, and greater mortality. These analyses controlled for several covariates, and results were in line with earlier findings from the Programs of All-Inclusive Care for the Elderly (PACE) setting9 and among ambulatory patients.10 Taken together, the MRS approach may be used to identify beneficiaries with high MRS and target them for risk-mitigating interventions.
For the Enhanced MTM model, BCBS-NPA is deploying the MRS in tandem with a clinical decision support system (CDSS) known as MedWise.11,12 This CDSS analyzes a beneficiary’s medication regimen in the context of the MRS risk factors, thereby identifying and resolving medication-related problems (MRPs) that are most likely contributing to the risk of ADEs and other negative utilization outcomes.6,7 Therefore, resolving such MRPs has the potential to address some of the performance variation associated with traditional MTM. However, what are those MRPs? When given access to the CDSS, how do pharmacists intervene on behalf of a PDP participating within the Enhanced MTM model? And, when comparing against the broader literature, will these interventions differ from those in traditional MTM?
The purpose of this study was to determine (1) the types of clinically actionable MRPs identifed by pharmacists who are intervening on behalf of a PDP that is participating in CMMI’s Enhanced MTM model, and (2) the types of recommendations made to resolve identified MRPs. The research presented here was conducted by Tabula Rasa HealthCare (TRHC) with the oversight of ClearStone Solutions (CSS), the contracted Part D administrator for plan participant BCBS-NPA, without input from CMS.
Methods
Study Design and Ethical Approvals
This is a retrospective, cross-sectional analysis of the MRPs identified by pharmacists for beneficiaries enrolled and targeted in Enhanced MTM plan years 2 and 3, which aligned with calendar years January 1, 2018, through December 31, 2019. The need for an informed consent procedure was waived by the Biomedical Research Alliance of New York Institutional Review Board (19-12-172-427; May 24, 2019).
Setting and Context
This study describes the Enhanced MTM model offered to beneficiaries living in Medicare Part D Region 25 (Iowa, Minnesota, Montana, Nebraska, North Dakota, South Dakota, and Wyoming), whose coverage was provided by BCBS-NPA.5 Through its Part D administrator, CSS, NPA partnered with TRHC, for provision of Enhanced MTM services. TRHC is a medication-risk mitigation and technology solutions company that focuses on optimizing medication regimens. This technology is utilized by trained and certified health care professionals (typically, pharmacists).
TRHC originally deployed the MRS and CDSS in the PACE setting. PACE is a value-based environment, where programs assume full financial risk of medically complex older adults dually eligible for Medicare and Medicaid. PACE pharmacists predominantly use TRHC technologies for prospective risk mitigation, where regimens can be optimized prior to ingestion (ie, at the point of prescribing/dispensing). Given TRHC’s extensive experience and success in PACE, CSS sought to determine whether TRHC’s technologies could be extrapolated to the larger Part D population using retrospective risk mitigation methods (ie, optimizing existing regimens) for Enhanced MTM.
Technology Description
The MRS ranges from 0 to 53, with higher scores representing greater risk of ADEs and other negative health utilization outcomes.6,7 For this analysis, high risk was defined as a score of 20 or higher.6,13 The MRS is based on 5 modifiable risk factors: anticholinergic burden, sedative burden, pharmacokinetic drug interactions, QT prolongation risk, and relative odds of ADEs as computed for the entire medication regimen using primary information obtained from the FDA’s Adverse Event Reporting System database.6,7
Whereas the CDSS is a separate and distinct tool, it works in tandem with the MRS. Specifically, it presents 4 visualizations of an individual’s medication regimen in the context of the aforementioned risk factors. The cornerstone of this tool is a comprehensive, matrix-based11 visual of the entire regimen. This helps pharmacists identify simultaneous multidrug interactions (SMDIs),14 such as competitive inhibition between 3 or more cytochrome P450 isoenzyme substrates. The tool also updates the archaic methodology of first assessing drug interactions as pairwise comparisons and then generating numerous clinical alerts.12,14 Clinical cases illustrating the CDSS matrix have been documented extensively.15-20
Intervention Description
BCBS-NPA’s intervention as a CMMI model participant was a pharmacist-provided medication safety review (MSR). MSRs were provided over the telephone by TRHC call-center pharmacists or face to face by community pharmacists. Unlike comprehensive medication reviews (CMRs)21 in traditional MTM, MSRs additionally“ (1) focus on applying the principles of pharmacodynamics, pharmacokinetics, pharmacogenomics (when available), and chronopharmacology to medication safety rather than being driven by CMS star ratings and medication and guideline adherence; and (2) involve evaluating [SMDIs] using [the] evidence-based, proprietary CDSS.”14 These 2 points are solely accomplished with a pharmacist’s expert review and interpretation of TRHC’s aforementioned CDSS matrix.
Prior to each program year, MRSs were computed for all beneficiaries using Part D claims data. Next, beneficiaries were stratified. Those with the highest risk received priority for MSR targeting; however, any beneficiary requesting an MSR could receive one (eg, a low-risk beneficiary whose spouse is at high risk).
Targeted beneficiaries were mailed documents describing the model. Pharmacy technicians, interns, or pharmacists then contacted the targeted beneficiaries or their designees (eg, caregiver) to gauge interest in an MSR consultation. If beneficiaries wished to participate, they first underwent a medication reconciliation process in which they reported all legend and non-legend medications and supplements. Staff documented the name, strength, dosage, and time of administration of each product and whether the beneficiary had any medication-related concerns.
Next, beneficiaries had an MSR consultation with a pharmacist. Prior to the MSR, pharmacists analyzed the medication regimen and identified actual or potential MRPs with assistance from the CDSS matrix. After formulating a prioritized problem list, pharmacists spoke with the beneficiary (or designee) about the MRP(s) and resolution strategies.
Documentation of MSR Consults
After the consultation, pharmacists provided the beneficiary/designee with a medication action plan (MAP) along with an updated medication list. The updates in the medication list reflected time-of-day administration changes that pharmacists made within their scope of practice14 to mitigate competitive inhibition.22 The MAP described the MRPs that were discussed and outlined the specific actions needed to resolve MRPs. With consent, pharmacists sent a prescriber’s action plan (PAP) to the prescribing physician by fax and/or mail. This contained (1) a 1- to 3-sentence executive summary of each MRP and its corresponding recommendation for resolution; (2) a lengthier rationale describing the recommendation’s evidence-based justification (if necessary); and (3) a hypothetical pre-post change in MRS after the implementation of the proposed recommendations.
Pharmacists recorded MSR details with Systematized Nomenclature of Medicine–Clinical Terms (SNOMED CT) codes. SNOMED CT is an internationally adapted, semantically rich, clinically validated, standardized medical terminology that permits consistent and accurate representation of clinical content in electronic medical records.23 All Enhanced MTM plan sponsors were required to transmit data to CMMI using SNOMED CT.24 When an MRP was encountered, pharmacists selected one SNOMED CT that described the nature of the MRP and another SNOMED CT that described a recommendation to resolve the MRP. The breadth of SNOMED CT also provided a way for pharmacists to document ancillary activities (eg, medication counseling, assessments) and problems that may not have been medication-related (eg, tobacco use, elder abuse, suicidal ideation). Such SNOMED CTs were operationally defined as “risk factors” and were not considered MRPs for this research. Once details were coded, pharmacists wrote a narrative description outlining the problems identified; the description was then given to the patient and/or prescriber.
Study Subjects
Subjects included all beneficiaries who received their first-ever MSR for plan years 2018 and 2019. First MSRs were included because the number of MRPs was likely to be greatest at the first consult.
Study Procedures and Definitions
The objective of this study was to report the frequencies of “clinically actionable” MRPs and recommendations. For this study, a “clinically actionable” MRP was defined as any SNOMED CT (1) that was included in a MAP or PAP; (2) that described an MRP; and (3) whose recommendation for resolution, if implemented, would result in a tangible alteration to the beneficiary’s medication regimen. Therefore, MRPs were not considered “clinically actionable” if “continue,” “monitor,” “educate,” or “referral” was the pharmacist’s recommended intervention. Excluded SNOMED CTs are reported in eAppendix Table 1 (eAppendix available here).
Upon examination of the SNOMED CTs, 60 unique MRPs and 19 unique recommendation codes were documented in MAPs and PAPs. To facilitate interpretation, we recategorized SNOMED CTs using broader taxonomies. For MRPs, we reclassified SNOMED CTs into 8 MRP categories described by Hepler and Strand.25 We renamed Hepler and Strand’s “failure to receive drug” as “adherence/cost problem” (eAppendix Table 2). For recommendations, we reclassified SNOMED CTs into recommendation categories described by Hoth et al (eAppendix Table 3).26
Analysis
We met our primary objectives by counting the number of MRPs and recommendations in each category across the MSRs. We performed several additional analyses. First, we identified medications causing or contributing to MRPs. Next, we examined whether significant differences existed between beneficiaries with and without clinically actionable MRPs. We used the unpaired t test to compare continuous, parametric data and the chi-square test to compare categorical data. Lastly, we examined the relationship between the MRS and the number of clinically actionable MRPs using a second-order polynomial regression weighted by the number of members at each MRS. Finally, a paired t test was used to evaluate the hypothetical change in MRS that would occur postconsultation, assuming 100% implementation. We considered P values < .05 statistically significant. Analyses were conducted using R (v. 3.5.1, 3.5.2, and 4.0.5) and Microsoft Excel (Microsoft 2019, Redmond, WA).
Results
There were 22,696 beneficiaries who received a first-ever MSR for plan years 2018 and 2019. Most beneficiaries (n = 18,703; 82.4%) had at least 1 clinically actionable MRP coded in their MAP and/or PAP (eAppendix Figure 1). Of these, the majority of MSRs (n = 15,643/18,703; 83.6%) were completed by call-center pharmacists, and the rest were completed by community pharmacists. Compared with the 3993 beneficiaries without clinically actionable MRPs, the group with actionable MRPs had a significantly higher mean (SD) MRS (19.0 [6.8] vs 15.6 [6.6]; P < .001), had a significantly greater proportion of high risk (ie, MRS ≥ 20) beneficiaries (44.1% vs 25.9%; P < .001), and took, on average, 2 more medications (mean [SD]:13.6 [5.0] vs 11.6 [4.8]; P < .001). Moreover, several medical conditions were more prevalent among beneficiaries with actionable MRPs. Table 1 reports all demographic details.
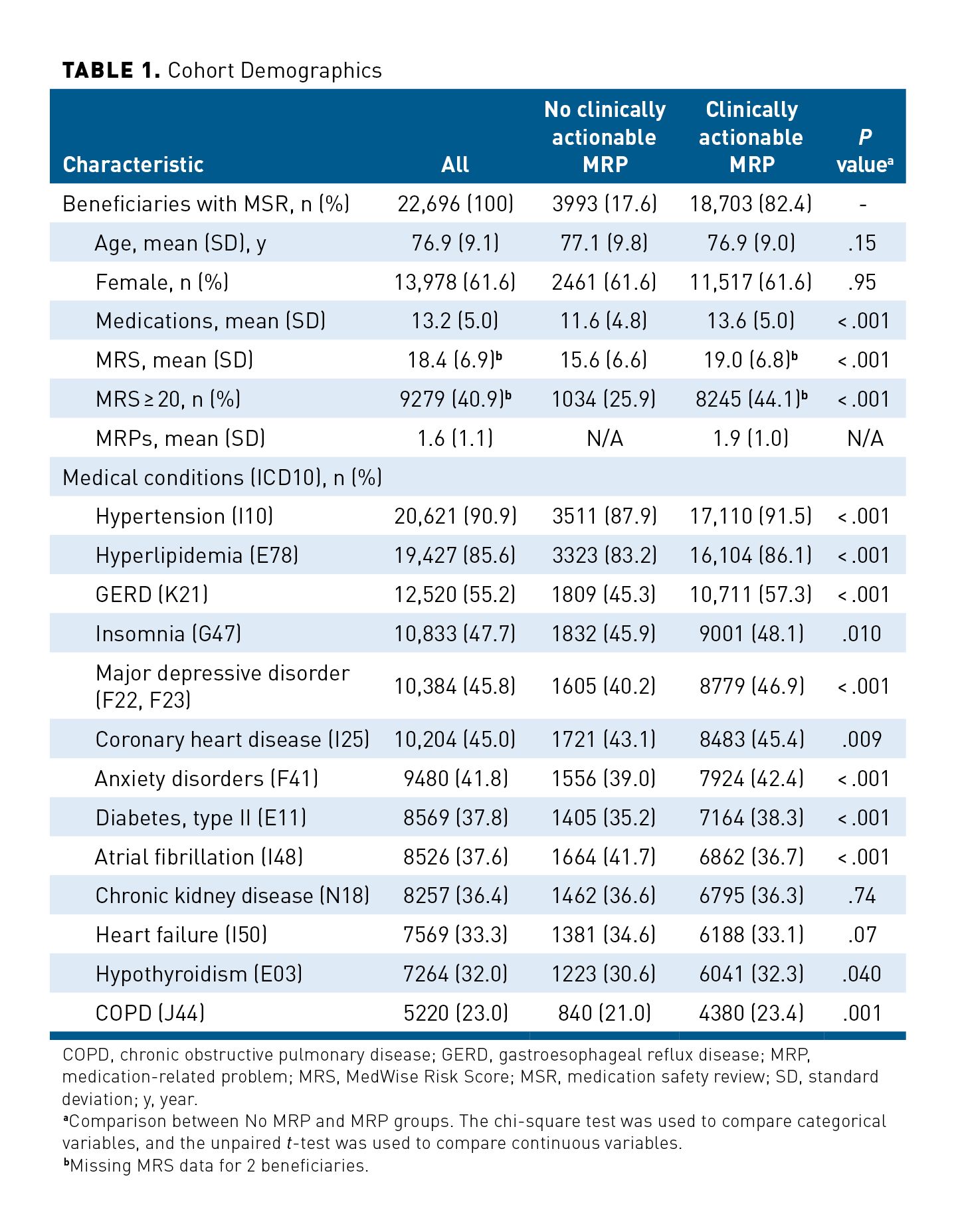
In total, pharmacists identified 36,455 MRPs, corresponding to a mean (SD) number of MRPs of 1.6 (1.1) across the whole population and 1.9 (1.0) for the 18,703 beneficiaries with actionable MRPs. A second-order polynomial regression equation showed that the MRS is positively associated with the number of clinically actionable MRPs identified (Figure 1; MRPs = 0.498 + 0.073 * MRS – 0.0006 * MRS2; adjusted R2 = 0.98, P < .001).
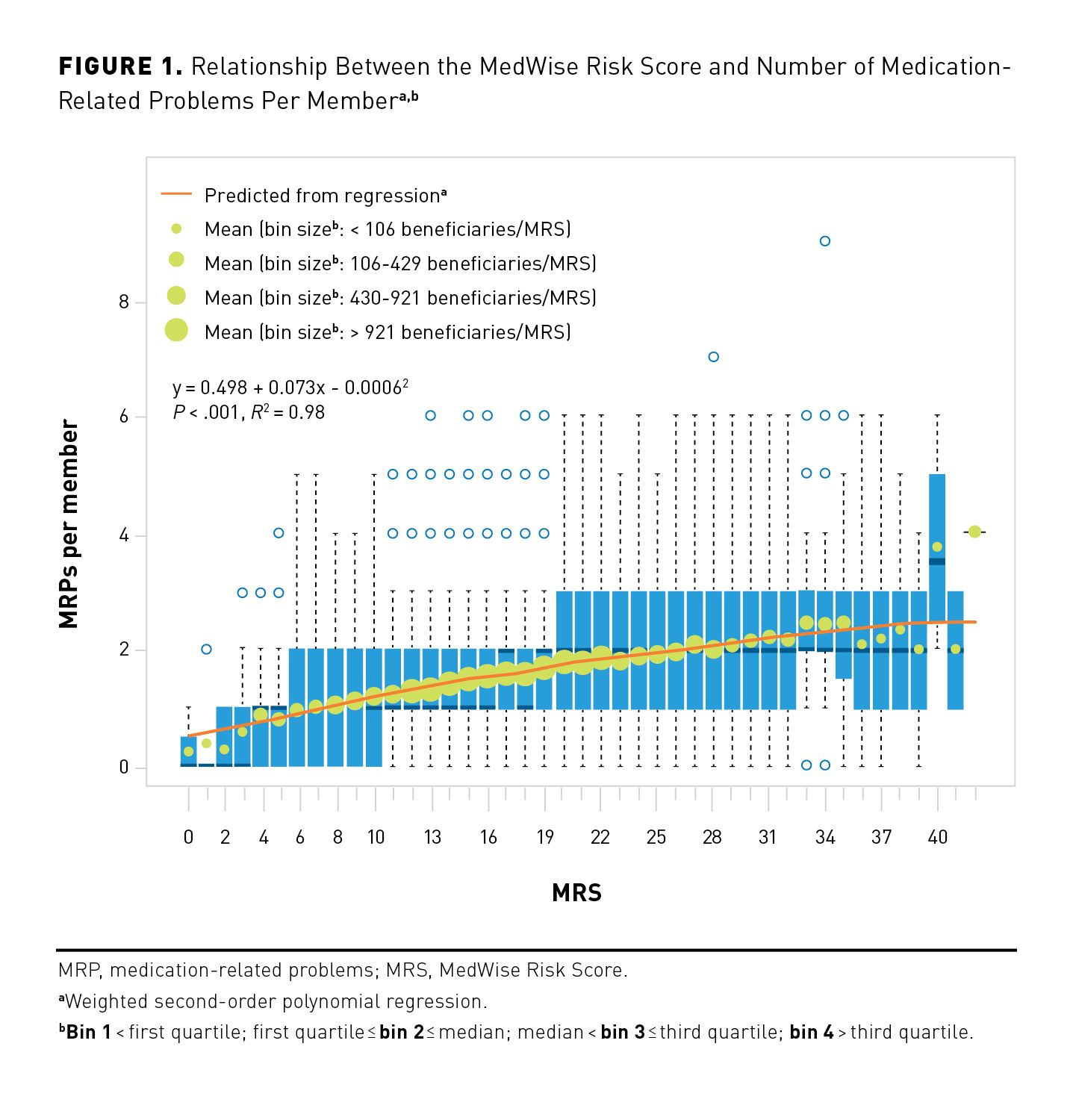
Figure 2A depicts the distribution of clinically actionable MRPs identified by pharmacists. “Adverse drug reaction” was the most common MRP identified, which represented 40.6% (n = 14,788) of all identified MRPs. “Drug interaction” (n = 9716; 26.7%) and “drug use without indication” (n = 6496; 17.8%) were the next most common MRPs. These 3 MRP categories accounted for 85.1% of all MRPs. To resolve MRPs, pharmacists most frequently recommended to “start alternative therapy” (n = 12,219; 33.5%), “discontinue medication” (n = 12,059; 33.1%), “change time of medication administration” (n = 6459; 17.7%), and “change dose” (n = 4393; 12.1%). Collectively, these 4 recommendations represented 96.4% of all recommendations. The overall distribution of recommendations can be seen in Figure 2B. Regarding the top 3 MRPs, pharmacists most frequently recommended to “start alternative therapy” to resolve “adverse drug reactions” (45.5%; 6724/14,788); to “change time of administration” to resolve “drug interactions” (53.4%; 5189/9716), and to “discontinue medication” to resolve “drug use without indication” (88.0%; 5718/6496). As shown in Figure 3, pharmacists’ recommendations could significantly decrease the average MRS by 4.7 points (95% CI, 4.6-4.8; P < .001).
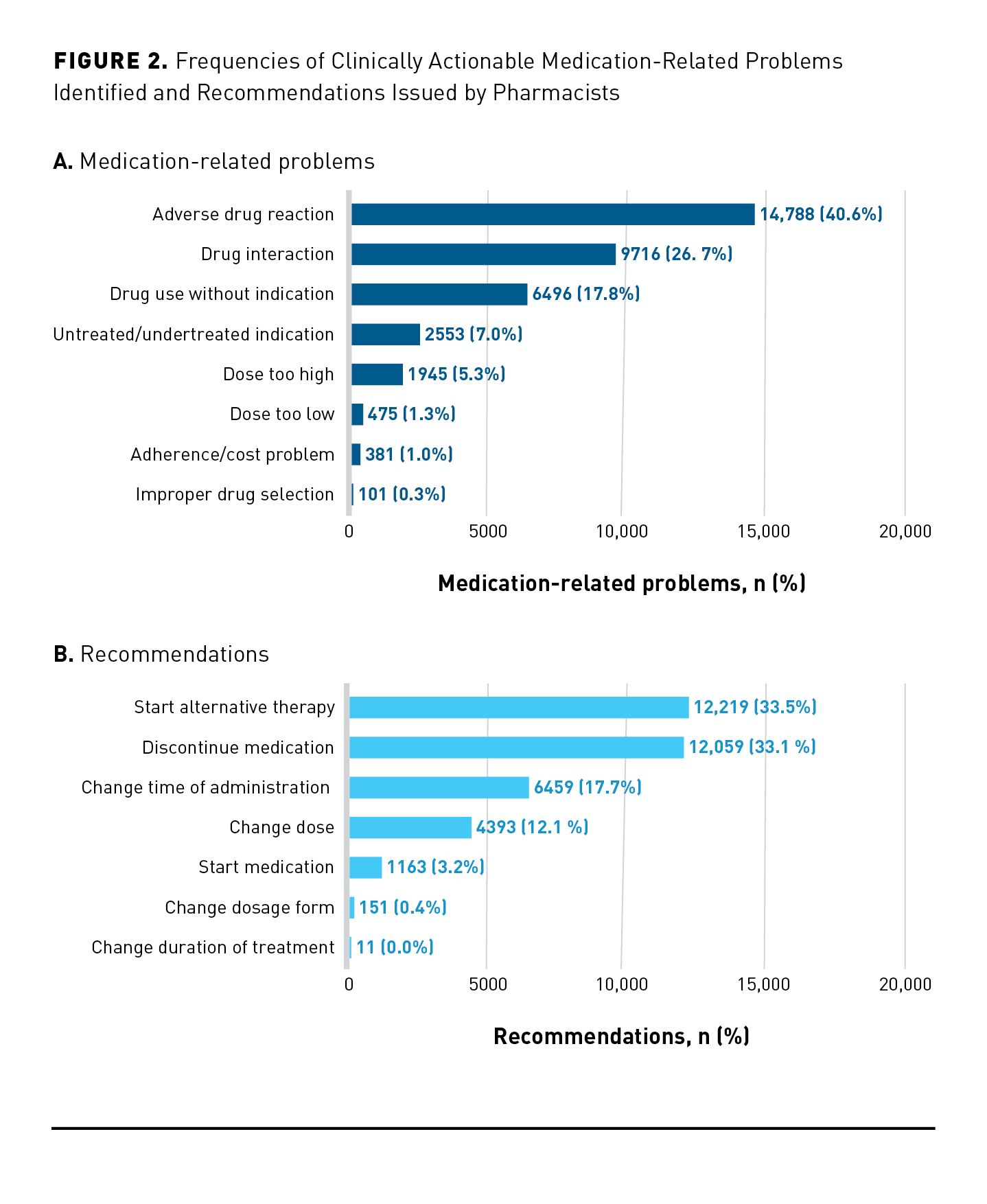
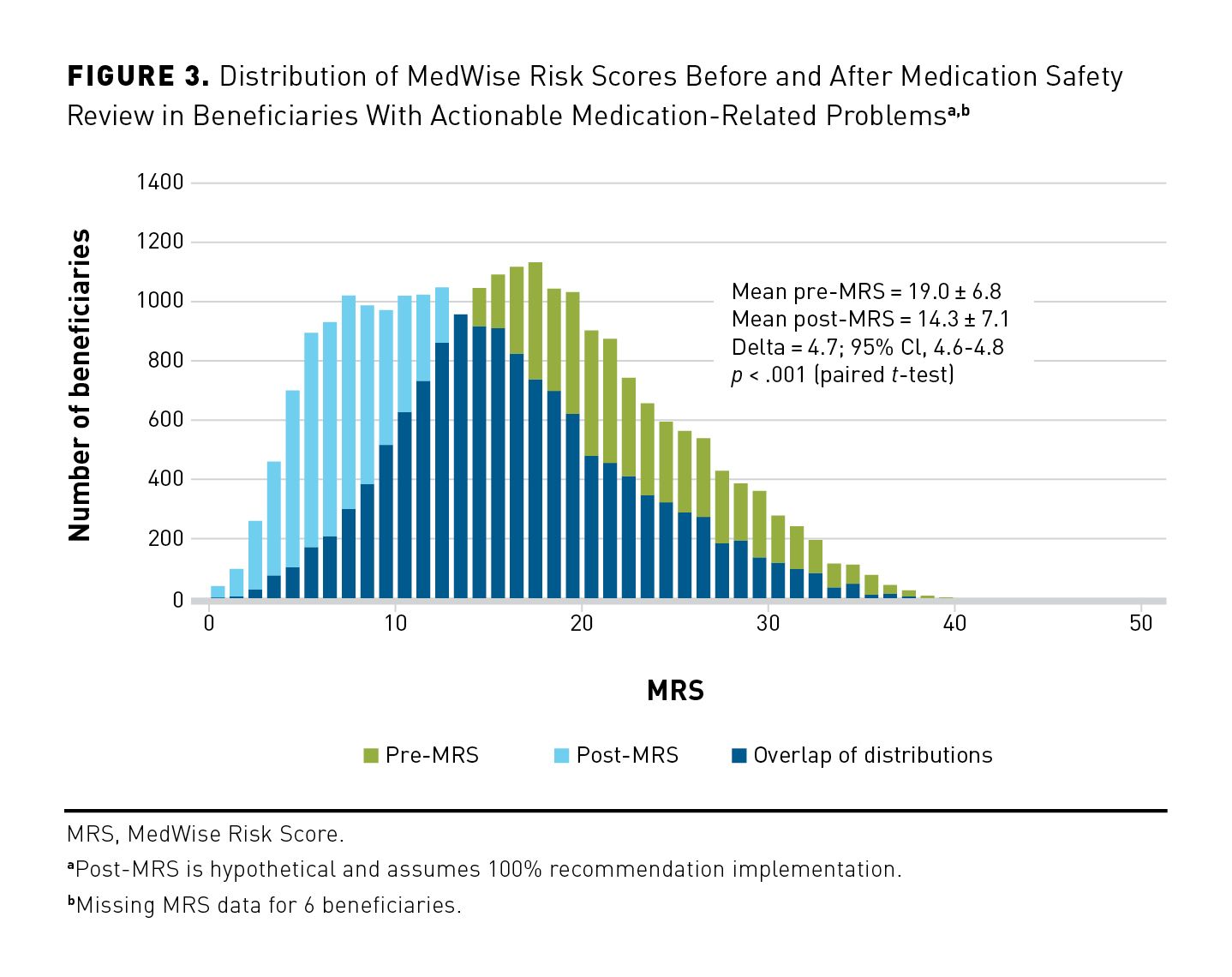
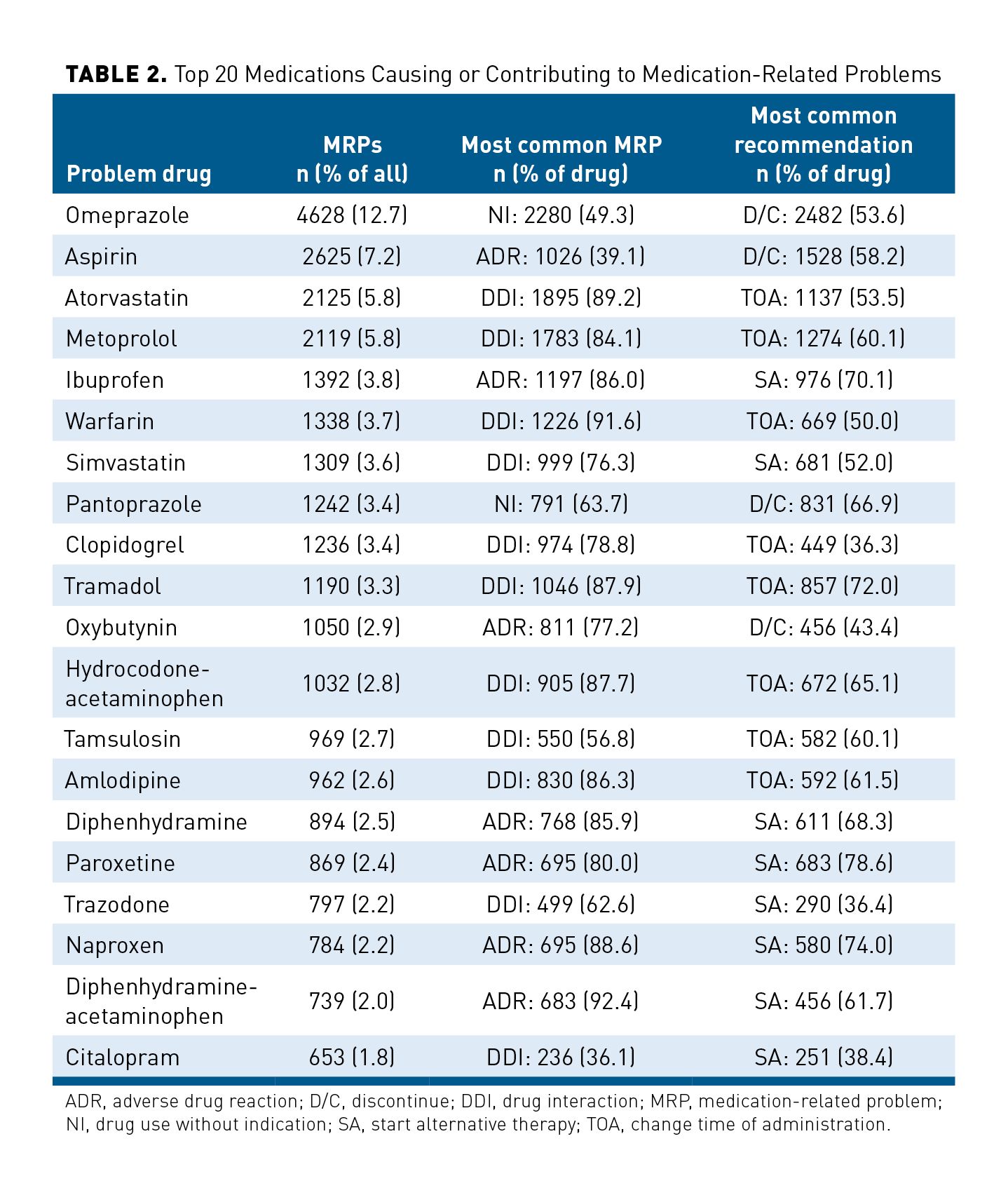
Table 2 reports the top 20 medications that were identified as causing or contributing to MRPs, along with the corresponding most commonly coded MRP and recommendation for resolution. For example, the most commonly identified problem medication was omeprazole, which was coded in 12.7% (4628/36,455) of all MRPs. Pharmacists most frequently found omeprazole not to be indicated (49.3%; 2280/4628) and most frequently recommended to have it discontinued (53.6%; 2482/4628). Pharmacists commonly coded aspirin, ibuprofen, diphenhydramine, diphenhydramine-acetaminophen, oxybutynin, paroxetine, and naproxen with “adverse drug reaction” SNOMED CTs. Furthermore, hydrocodone-acetaminophen, amlodipine, tramadol, warfarin, clopidogrel, simvastatin, atorvastatin, metoprolol, tamsulosin, trazodone, and citalopram were commonly coded with “drug interaction” SNOMED CTs.
Discussion
This study examined nearly 23,000 beneficiaries who received their first-ever MSR, which was the primary intervention deployed by a PDP participating in CMMI’s ongoing Enhanced MTM model. Across 2 program years, clinically actionable MRPs were identified among 82.4% of beneficiaries, with about 2 such MRPs identified per beneficiary. Three MRPs pertaining to medication safety (ie, adverse drug reactions, drug interactions, and drug use without indication) accounted for approximately 85% of all MRPs. At roughly similar rates (~33% each), pharmacists recommended deprescribing or changing drugs to resolve MRPs. Further, we found that the MRS was positively associated with the number of MRPs, and, if recommendations were implemented, the MRS would decrease. Collectively, these results suggest that risk stratification worked as intended: Pharmacists were able to identify a substantial number of actionable MRPs that may cause or contribute to ADEs and then act accordingly to decrease risk.
Whereas this is not the only MTM model to identify medication safety–oriented problems,27-31 other programs have described very different priorities. For example, a 2019 study of 80,000 Medicare patients enrolled in an MTM program at Humana found that, depending on the intervention year, nonadherence made up 50% to 90% of the MRPs.32 Drawing from 10 years of MTM experience, an integrated health system from Minnesota identified medication underuse to be the predominant problem, which manifested in “need for new medication” (28%), “subtherapeutic doses” (26%), and “nonadherence” (17%).33 Another multistate, 7-year study found cost-efficacy management, medication underuse, and suboptimal therapy to be among the most common MTM priorities noted in the community pharmacy setting.34 Similarly, several other MTM programs describe nonadherence, undertreatment, and medication cost as the main clinical priorities.35-43
We contend that the medication safety and deprescribing focus reported here is related to the advanced CDSS deployed by this PDP as part of its participation within the enhanced MTM model. A small pilot study assessed the clinical behavior of traditional MTM pharmacists when presented simulated cases of polymedicated patients (≥ 10 medications in profile).44 Prior to CDSS exposure and training, pharmacists most frequently recommended “start new medication,” which reflected their program’s operational focus on disease-state guideline adherence. After training, pharmacists were significantly more likely to recommend deprescribing and changing medications, which reflected a new focus on ADE-risk mitigation.44 When the CDSS was used for the care of about 1000 medically complex older adults enrolled in PACE, pharmacists similarly prioritized MRPs related to medication safety: “drug interactions” (36%), “adverse drug reactions” (18%), “high doses” (14%), and “drug use without indication” (13%). In line with the present study, PACE pharmacists most commonly recommended deprescribing, changing medications, and changing times of administration to resolve MRPs.14
Medication safety–focused interventions could help lower hospital and outpatient costs. Among older adults, ADEs may contribute to 12% of hospital admissions45 and cause 1.2 million annual outpatient visits in the United States.46 Since ADEs are largely preventable,47 associated hospital and outpatient costs can likely be avoided. In this study, pharmacists prioritized high-risk medications that are associated with these negative outcomes. For example, opioids (eg, hydrocodone and tramadol), anticoagulants (eg, warfarin), antiplatelets (eg, clopidogrel, aspirin), antidepressants (eg, paroxetine), nonsteroidal anti-inflammatory drugs (eg, ibuprofen), and anticholinergics (eg, diphenhydramine, oxybutynin) are frequent culprits in drug-related hospitalizations and injurious falls.45,48-50 The next article51 in this supplement will illustrate how these interventions can indeed yield lower Parts A and B costs.
Limitations
The study has 4 main limitations. First, the distribution of MRPs and recommendations is dependent on pharmacists’ coding consistency and quality. This may be most relevant when a given MRP or recommendation may be coded in 2 different ways. For example, aspirin 81 mg used for primary prevention of cardiovascular disease in a 85-year-old person with low cardiovascular risk could be reasonably documented with “at risk of medication side effect” (ie, bleeding) or “medication therapy unnecessary.”52 Miscoding could also impact the extent to which the MRS can hypothetically change after 100% implementation of recommendations. Extensive and repeated training provided to pharmacists participating in this program and continual verification and validation may have minimized the impact of this limitation. Second, recategorizing SNOMED CT codes into broader and different MRPs and recommendation taxonomies may have inadvertently biased distributions. However, expert opinion used in the recategorization process should have limited such an impact. Third, MedWise cannot account for “composite” recommendations that may influence the interpretation of pharmacists’ activities. For example, if a drug interaction is identified as the MRP, then a pharmacist may provide the doctor with 2 recommendation options to resolve the interactions: (1) discontinuing the perpetrator drug or (2) decreasing the dose of the victim drug. Whereas both options are written as a narrative description within the MAP and/or PAP, the pharmacist would have to pick 1 SNOMED CT recommendation option to code into the action plan. Fourth and finally, this study’s dataset did not capture recommendation implementation. Future studies should capture this intermediate outcome because it can help quantify the feasibility of MSR interventions. Nevertheless, the next article51 in this supplement will show that several utilization outcomes improved in the year following the receipt of a first-ever MSR, indicating that recommendations were feasible by dint of their effectiveness.
Conclusions
Adverse drug reactions, drug interactions, and unnecessary medication use accounted for the majority of MRPs identified by pharmacists who used an advanced CDSS, MedWise, to support one PDP’s participation within CMMI’s Enhanced MTM model. To resolve these medication safety–oriented problems, pharmacists issued recommendations capable of significantly decreasing the MRS, such as changing or deprescribing medications. These MedWise-supported medication safety interventions may augment traditional MTM programs with different priorities (eg, nonadherence, undertreatment, medication cost). The impact of medication safety–oriented interventions on economic and clinical outcomes will be illustrated in the next article.51
Acknowledgements
The authors would like to thank Dr Calvin Knowlton and Dr Orsula Knowlton for their forward-looking vision and unequivocal, lifelong dedication to the advancement of the pharmacy profession; Brian Litten for legal counsel and support of the program; Steve Gilbert for supporting operational and clinical quality; Beth Eichfeld for her involvement as program manager; Reginald Williams, Harold Escarrilla, and Sue Yang for assistance in preparing comorbidity, drug, and proposed risk score data; Stephanie Carletti for her unwavering support of the program, facilitating operational efficiency and success; the following pharmacists for their sincere efforts to improve the safety, appropriateness, and quality of the Enhanced MTM beneficiaries’ medication regimens: Amanda Mastrogiovanni, Courtney Christopher, Craig Rein, Daniel Green, Danielle Cantalupo, Darria Zangari, Debbie Lukose, Desiree Massari, Dina Amer, Drusty Bavishi, Gabriella Latorre, JT Skelly, Jennifer Metzler, Jessica Growette, Joseph Wood, Kaitlin Savona, Kirsten Demarco, Kirsten Gallo, Lauren Petroski, Loren Martinez, Megan Lew, Melissa Metzger, Natalie Ferro, Neil Patel, Palak Gohil, Richard Chang, Scott Raymond, Kevin Bain, and Valerie Buchanan; the following technicians for their strong efforts in engaging beneficiaries and obtaining accurate medication lists: Abigail Malone, Alayna Marucci, Amanda Seals, Amber Mosteller, Andrew Palmer, Ashley Approvato, Briana Joseph, Brittany Connelly, Christina Bean, Donna Van Staveren, Emlyn Williams, Eva Duarte, Gabrielle Parker, Gregg Eppleman, Heather Green, Heather Klarmann, Helen Martin, Jaime Picchi, Jaimie Doyle, Jamie Kilmurray, Jeanna Comorote, Jonquil Steele, Josselyne Duarte, Kimberly Shaw, Krista Maniaci, Layla Pineda, Lindsey Rehberger, Maureen Molzon, Melissa Jorge, Naomi Colon, Rachael Carpenter, Raquel Rivera, Samantha O’Connell, Stacie Riker, Starr Santiago, Tara Russo, and Victor Vo; the following outreach liaisons for their strong efforts in engaging beneficiaries: Ed Demetreshon, Joan Greenhalgh, Linda Moore, and Samantha Sklikas; the following individuals from ClearStone: Jessica Growette, Lucas Castillo, Jenny Steinke, Bonnie Haukom, Sarah Legatt, Nick Jejel, Paul Fischer, Angela Mitchell, and Nancy Sheehan. We also thank Dana Filippoli for the review of the manuscript.
Author affiliations: Office of Translational Research and Residency Programs, Tabula Rasa HealthCare, Moorestown, NJ (DB, KP); Office of Healthcare Analytics, Tabula Rasa HealthCare, Moorestown, NJ (SF); Office of Applied Pharmacotherapy, Tabula Rasa HealthCare, Moorestown, NJ (MSA); Office of Healthcare Analytics, Tabula Rasa HealthCare, Moorestown, NJ (AS); ClearStone Solutions, Government Markets Division, Blue Cross and Blue Shield of Minnesota, Eagan, MN(JJ); Tabula Rasa HealthCare, Orlando, FL, and Université de Montréal, Montréal, Québec, Canada (JT).
Funding source: Funding was obtained by Blue Cross and Blue Shield Northern Plains Alliance (NPA) in Medicare Part D Region 25 (IA, MN, MT, ND, NE, SD, and WY), the model participant, under the 5-year CMS Innovation Center’s Enhanced Medication Therapy Management (MTM) model. Through its NPA’s Part D plan administrator, ClearStone Solutions, funds were provided to Tabula Rasa HealthCare for delivery of Enhanced MTM services and research. In this study, data were analyzed regardless of patient’s enrollment in the Enhanced MTM program. This supplement was supported by Tabula Rasa HealthCare.
Author disclosures: Drs Bankes, Pizzolato, Finnel, Awadalla, Stein, and Turgeon report employment and stock ownership with Tabula Rasa HealthCare, and ownership of the MedWise Risk Score used in this study. Dr Johnson reports employment with ClearStone Solutions (an affiliate of Blue Cross andBlue Shield of Minnesota), which administers the Enhanced MTM Pilot on behalf of Blue Cross and Blue Shield Northern Plains Alliance. ClearStone Solutions paid Tabula Rasa HealthCare to provide the Enhanced MTM services described. Dr Turgeon reports patents received (10,890,577: Methods of treatment having reduced drug-related toxicity and methods of identifying the likelihood of patient harm arising from prescribed medications) and patents pending (Methods of treatment having reduced drug-related toxicity and methods of identifying the likelihood of patient harm arising from prescribed medications: 17/143,936; Population-based medication risk stratification and personalized medication risk score: 16/870,517).
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies. The research presented here was conducted by the model participant. Findings might or might not be consistent with or confirmed by the findings of the independent evaluation contractor. CMS considers any findings from the Part D Enhanced MTM model to be preliminary until certified as valid by its Research and Rapid Cycle Evaluation Group (RREG).
Authorship information: Concept and design (DB, SF, JT); acquisition of data (DB, SF, MSA); analysis and interpretation of data (DB, KP, SF, AS); drafting of the manuscript (DB, KP, SF, JT); critical revision of the manuscript for important intellectual content (DB, KP, SF, MSA, AS, JJ, JT); statistical analysis (DB, SF); provision of study materials or patients (JJ); obtaining funding (JJ); administrative, technical, or logistical support (DB, SF, MSA, AS, JJ, JT); and supervision (JT).
Address correspondence to:Jacques Turgeon, PhD, 13485 Veterans Way, Ste 410, Orlando, FL 32827. E-mail: jturgeon@trhc.com
REFERENCES
1. Pellegrino AN, Martin MT, Tilton JJ, Touchette DR. Medication therapy management services: definitions and outcomes. Drugs. 2009;69(4):393-406. doi:10.2165/00003495-200969040-00001
2. Perlroth D, Marrufo G, Montesinos A, et al. Medication therapy management in chronically ill populations: final report. Published August 2013. Accessed June 22, 2021. https://innovation.cms.gov/files/reports/mtm_final_report.pdf
3. Gray C, Cooke CE, Brandt N. Evolution of the Medicare Part D medication therapy management program from inception in 2006 to the present. Am Health Drug Benefits. 2019;12(5):243-251. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6979045/pdf/ahdb-12-243.pdf
4. Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76-87. doi:10.1001/jamainternmed.2014.5841
5. Centers for Medicare & Medicaid Services. Part D enhanced medication therapy management model. Accessed June 22, 2021. https://innovation.cms.gov/innovation-models/enhancedmtm
6. Cicali B, Michaud V, Knowlton CH, Turgeon J. Application of a novel medication-related risk stratification strategy to a self-funded employer population. Accessed June 23, 2021. https://www.carekinesis.com/wp-content/uploads/2018_Cicali_B_Benefits_Q_Application_of_a_novel_medication-related_risk_stratification_strategy.pdf
7. Turgeon J, Michaud V, Cicali B, inventors. Patent WO2019089725 - Population-based medication risk stratification and personalized medication risk score. World Intellectual Property Organization. September 5, 2019. Accessed June 23, 2021. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019089725
8. Michaud V, Smith MK, Bikmetov R, et al. Association of a medication risk score with health care outcomes. Am J Manag Care. 2021;27(suppl 16):S280-S291.
9. Bankes DL, Jin H, Finnel S, et al. Association of a novel medication risk score with adverse drug events and other pertinent outcomes among participants of the programs of all-inclusive care for the elderly. Pharmacy (Basel). 2020;8(2):87. doi:10.3390/pharmacy8020087
10. Ratigan AR, Michaud V, Turgeon J, et al. Longitudinal association of a medication risk score with mortality among ambulatory patients acquired through electronic health record data. J Patient Saf. 2021;17(4):249-255. doi:10.1097/PTS.0000000000000829
11. Knowlton CH. Medication risk mitigation matrix: a pharmaceutical care opportunity for precision medication. J Am Pharm Assoc (2003). 2015;55(4):354-358. doi:10.1331/JAPhA.2015.15527
12. Turgeon J, Michaud V. Clinical decision support systems: great promises for better management of patients’ drug therapy. Expert Opin Drug Metab Toxicol. 2016;12(9):993-995. doi:10.1517/17425255.2016.1171317
13. Al Rihani SB, Smith MK, Bikmetov R, et al. Risk of adverse drug events following the virtual addition of COVID-19 repurposed drugs to drug regimens of frail older adults with polypharmacy. J Clin Med. 2020;9(8):2591-2609. doi:10.3390/jcm9082591
14. Bankes DL, Amin NS, Bardolia C, Awadalla MS, Knowlton CH, Bain KT. Medication-related problems encountered in the program of all-inclusive care for the elderly: an observational study. J Am Pharm Assoc (2003). 2020;60(2):319-327. doi:10.1016/j.japh.2019.10.012
15. Matos A, Bankes DL, Bain KT, Ballinghoff T, Turgeon J. Opioids, polypharmacy, and drug interactions: a technological paradigm shift is needed to ameliorate the ongoing opioid epidemic. Pharmacy (Basel). 2020;8(3):154-172. doi:10.3390/pharmacy8030154
16. Bain KT, McGain D, Cicali EJ, Knowlton CH, Michaud V, Turgeon J. Precision medication: an illustrative case series guiding the clinical application of multi-drug interactions and pharmacogenomics. Clin Case Rep. 2019;8(2):305-312. doi:10.1002/ccr3.2604
17. Tranchina K, Turgeon J, Bingham J. Integrating a novel medication risk score and use of an advanced clinical decision support system into a pharmacist- and nurse-coordinated transition of care program to mitigate drug interactions. Clin Case Rep J. 2021;2(1):1-5.
18. Bardolia C, Michaud V, Turgeon J, Amin NS. Deprescribing dual therapy in benign prostatic hyperplasia: a patient case. Case Reports. 2020;10(5). doi:10.37421/jccr.2020.10.1349
19. Ballinghoff T, Bain K, Matos A, Bardolia C, Turgeon J, Amin NS. Opioid response in an individual with altered cytochrome P450 2D6 activity: implications of a pharmacogenomics case. Clin Case Rep J. 2020;1(6):1-4.
20. Bain KT, Knowlton CH, Turgeon J. Medication risk mitigation: coordinating and collaborating with health care systems, universities, and researchers to facilitate the design and execution of practice-based research. Clin Geriatr Med. 2017;33(2):257-281. doi:10.1016/j.cger.2017.01.009
21. Miller DE, Roane TE, Salo JA, Hardin HC. Evaluation of comprehensive medication review completion rates using 3 patient outreach models. J Manag Care Spec Pharm. 2016;22(7):796-800. doi:10.18553/jmcp.2016.22.7.796
22. Deodhar M, Al Rihani SB, Arwood MJ, et al. Mechanisms of CYP450 inhibition: understanding drug-drug interactions due to mechanism-based inhibition in clinical practice. Pharmaceutics. 2020;12(9):846. doi:10.3390/pharmaceutics12090846
23. Millar J. The need for a global language - SNOMED CT introduction. Stud Health Technol Inform. 2016;225:683-685.
24. Barlas S. CMS to test enhanced medication therapy management model: aims for greater use of pharmacists, cost savings, and better outcomes. P T. 2016;41(7):423-441. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4927016/pdf/ptj4107423.pdf
25. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533-543.
26. Hoth AB, Carter BL, Ness J, et al. Development and reliability testing of the clinical pharmacist recommendation taxonomy. Pharmacotherapy. 2007;27(5):639-646. doi:10.1592/phco.27.5.639
27. Touchette DR, Masica AL, Dolor RJ, et al. Safety-focused medication therapy management: a randomized controlled trial. J Am Pharm Assoc (2003). 2012;52(5):603-612. doi:10.1331/JAPhA.2012.12036
28. Welch EK, Delate T, Chester EA, Stubbings T. Assessment of the impact of medication therapy management delivered to home-based Medicare beneficiaries. Ann Pharmacother. 2009;43(4):603-610. doi:10.1345/aph.1L524
29. Almodóvar AS, Nahata MC. Associations between chronic disease, polypharmacy, and medication-related problems among Medicare beneficiaries. J Manag Care Spec Pharm. 2019;25(5):573-577. doi:10.18553/jmcp.2019.25.5.573
30. Zillich AJ, Snyder ME, Frail CK, et al. A randomized, controlled pragmatic trial of telephonic medication therapy management to reduce hospitalization in home health patients. Health Serv Res. 2014;49(5):1537-1554. doi:10.1111/1475-6773.12176
31. Caffiero N, Delate T, Ehizuelen MD, Vogel K. Effectiveness of a clinical pharmacist medication therapy management program in discontinuation of drugs to avoid in the elderly. J Manag Care Spec Pharm. 2017;23(5):525-531. doi:10.18553/jmcp.2017.23.5.525
32. Ferries E, Dye JT, Hall B, Ndehi L, Schwab P, Vaccaro J. Comparison of medication therapy management services and their effects on health care utilization and medication adherence. J Manag Care Spec Pharm. 2019;25(6):688-695. doi:10.18553/jmcp.2019.25.6.688
33. Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16(3):185-195. doi:10.18553/jmcp.2010.16.3.185
34. Barnett MJ, Frank J, Wehring H, et al. Analysis of pharmacist-provided medication therapy management (MTM) services in community pharmacies over 7 years. J Manag Care Pharm.
2009;15(1):18-31. doi:10.18553/jmcp.2009.15.1.18
35. Doucette WR, McDonough RP, Klepser D, McCarthy R. Comprehensive medication therapy management: identifying and resolving drug-related issues in a community pharmacy. Clin Ther. 2005;27(7):1104-1111. doi:10.1016/s0149-2918(05)00146-3
36. Moczygemba LR, Barner JC, Lawson KA, et al. Impact of telephone medication therapy management on medication and health-related problems, medication adherence, and Medicare Part D drug costs: a 6-month follow up. Am J Geriatr Pharmacother. 2011;9(5):328-338. doi:10.1016/j.amjopharm.2011.08.001
37. Perera PN, Guy MC, Sweaney AM, Boesen KP. Evaluation of prescriber responses to pharmacist recommendations communicated by fax in a medication therapy management program (MTMP). J Manag Care Pharm. 2011;17(5):345-354. doi:10.18553/jmcp.2011.17.5.345
38. Took RL, Liu Y, Kuehl PG. A study to identify medication-related problems and associated cost avoidance by community pharmacists during a comprehensive medication review in patients one week post hospitalization. Pharmacy (Basel). 2019;7(2):51-59. doi:10.3390/pharmacy7020051
39. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-214. doi:10.1331/JAPhA.2008.07108
40. Truong HA, Groves CN, Congdon HB, Dang DTT, Botchway R, Thomas J. Potential cost savings of medication therapy management in safety-net clinics. J Am Pharm Assoc (2003). 2015;55(3):269-272. doi:10.1331/JAPhA.2015.14062
41. Corsi K, Lemay V, Orr KK, Cohen L. Pharmacist medication therapy management in home health care: investigation of a sustainable practice model. J Am Pharm Assoc (2003). 2018;58(4S):S64-S68. doi:10.1016/j.japh.2018.04.028
42. Brophy L, Williams A, Berman EJ, et al. Collaborative DTM reduces hospitalization and healthcare costs in patients with diabetes treated with polypharmacy. Am J Manag Care. 2014;20(3):e72-e81.
43. Pindolia VK, Stebelsky L, Romain TM, Luoma L, Nowak SN, Gillanders F. Mitigation of medication mishaps via medication therapy management. Ann Pharmacother. 2009;43(4):611-620. doi:10.1345/aph.1L591
44. Bingham JM, Michaud V, Turgeon J, Axon DR. Effectiveness of an advanced clinical decision support system on clinical decision-making skills in a call center medication therapy management pharmacy setting: a pilot study. Pharmacy (Basel). 2020;8(4):228. doi:10.3390/pharmacy8040228
45. Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR. Hospitalization in older patients due to adverse drug reactions-the need for a prediction tool. Clin Interv Aging. 2016;11:497-505. doi:10.2147/CIA.S99097
46. Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19(9):901-910. doi:10.1002/pds.1984
47. Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions—a meta-analysis. PLoS One. 2012;7(3):e33236. doi:10.1371/journal.pone.0033236
48. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency
department visits for outpatient adverse drug events, 2013-2014. JAMA. 2016;316(20):2115-2125. doi:10.1001/jama.2016.16201
49. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012. doi:10.1056/NEJMsa1103053
50. Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013-1019. doi:10.1001/archinternmed.2011.215
51. Stein A, Finnel S, Bankes, D, et al. Health outcomes from an innovative enhanced medication therapy management strategy. Am J Manag Care. 2021;27(suppl 16):S300-S308.
52. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
2 Commerce Drive
Cranbury, NJ 08512
AJMC®
All rights reserved.
