- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Managed Care Considerations of Weight Management Interventions for Obesity
ABSTRACT
The growing prevalence of obesity in the United States has presented an opportunity to increase knowledge about optimal treatment approaches based on a better understanding of patient and provider biases, health care coverage and practices, and social determinants of health. Guideline-recommended obesity treatment begins with lifestyle intervention, and weight management may be enhanced by metabolic and bariatric surgery or anti-obesity medication (AOM) use. However, patient and provider perceptions surrounding obesity and different treatment modalities may present barriers to discussion and uptake of these interventions. Furthermore, it is uncommon for all effective obesity treatments (particularly AOMs) to be covered by insurance. Limited patient access to these treatments carries the potential for negative health consequences and higher health care costs. For these reasons, managed care decision makers are encouraged to improve access to effective obesity treatments, including coverage of AOMs such as semaglutide 2.4 mg.
Am J Manag Care. 2022;28(suppl 15):S307-S318. doi:10.37765/ajmc.2022.89294
For author information and disclosures, see end of text.
Introduction
A 2013 resolution passed by the American Medical Association House of Delegates recognized obesity as a chronic medical disease and recommended that providers receive “appropriate financial support and payment from third-party payers” for the medical management of obesity and overweight.1 Since then, the age-adjusted prevalence of obesity among US adults 20 years or older increased from 37.7% in 2013-2014 to 42.4% in 2017-2018.2 With the rising prevalence of obesity comes the greater importance of treatments to combat this serious chronic disease. However, barriers in perception and practice and social determinants of health may hinder access to obesity interventions for clinically meaningful weight loss. Other articles in this supplement cover the extensive body of literature on obesity, including current medical understanding of obesity as a serious, chronic, and treatable disease; the clinical, economic, and patient well-being burdens associated with obesity; the guideline-recommended standard of care in the management of overweight and obesity; and the Semaglutide Treatment Effect in People with obesity (STEP) clinical trials of semaglutide 2.4 mg as a pharmacologic treatment for obesity.3-6 This article will discuss barriers and opportunities, from a managed care perspective, for achieving clinically impactful weight loss in individuals with excess adiposity.
Clinical Targets for Obesity Treatment
Effective obesity treatment may include lifestyle intervention, metabolic and bariatric surgery, and use of anti-obesity medications (AOMs).7,8 Marc-Andre Cornier, MD, further explores guideline recommendations for treatment of obesity and weight-related comorbidities in the third article of this supplement.5 Regardless of treatment modality, relatively small reductions in body weight can be effective in achieving clinically significant therapeutic goals for managing obesity and associated diseases.7,9
In 2016, the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) published clinical practice guidelines for the medical care of patients with obesity based on clinical characteristics and disease burden.7 According to these guidelines, reducing body weight by approximately 5% to 15% in individuals with a body mass index (BMI) of at least 25 kg/m2 can be sufficient for preventing or delaying the onset of type 2 diabetes (T2D) in at-risk individuals or for inducing clinically meaningful improvements in patients with other weight-related diseases.7 This guidance reinforces a report from a 2014 expert multidisciplinary working group panel organized by the National Institutes of Health citing medical evidence that weight loss of 5% to 10% can produce clinically significant health improvements.9 The panel noted that maintenance of long-term weight loss and associated downstream benefits on comorbidities is more likely when there is a greater degree of weight loss during the initial intervention period.9 Thus, even small amounts of weight loss in individuals with obesity or overweight can be associated with clinically meaningful improvements, and greater degrees of weight loss may confer larger health benefits.8
A full exploration of the effects of weight loss on health outcomes in patients with excess adiposity is beyond the scope of this article. However, decreased weight can also reduce the risks of, and improve health outcomes related to, comorbidities other than metabolic disorders and cardiovascular disease (CVD). For example, modest weight loss in patients with obesity and overweight is linked to a decreased risk of total knee replacement in osteoarthritis, increased asthma control, and reduced reflux symptoms and esophageal acid exposure in gastroesophageal reflux disease.7,10-12
Efficacy and Safety of Recommended Obesity Treatment Modalities
A variety of inherited and external factors (eg, genetics, family history, ethnicity, cultural practices, environment, use of certain prescribed medications) can put a person at increased risk for obesity and weight gain.7,13 Treatment guidelines for obesity recommend several approaches to weight management; these involve lifestyle intervention and, when lifestyle intervention alone is insufficient, additional treatment modalities, such as pharmacologic or surgical therapies.7,8 This article briefly covers the vast literature on safety and efficacy of recommended obesity interventions, focusing mainly on the managed care considerations of obesity treatment.
Lifestyle Intervention
Lifestyle intervention represents the initial standard of care for obesity treatment among patients with overweight or obesity; it also is an essential component of surgical and pharmacologic interventions used in patients who require additional treatment. The 3 primary facets of lifestyle intervention are reduced caloric intake, increased physical activity, and behavioral modifications.7,13
The US Preventive Services Task Force (USPSTF) completed a systematic review of data (published by August 2018) concerning the benefits and harms of interventions for weight loss and maintenance in adults with overweight and obesity.14 Across 89 trials, patients who received lifestyle intervention generally lost more weight, were more likely to achieve at least 5% weight loss, and maintained greater weight loss for up to 36 months. Rates of adverse events (AEs) often were not reported in these trials, yet the review found no serious harm related to lifestyle intervention.14 A separate meta-analysis of randomized clinical trials (RCTs) from 1980 through 2018 examined the results of lifestyle intervention in adults with obesity and overweight.15 Results from 31 trials involving a wide variety of lifestyle interventions showed that weight loss was greater in the intervention groups than in the control groups at 1 year, although the difference had decreased somewhat by 3 years. The study authors noted that reliable information about the impact of lifestyle intervention on mortality was difficult to gather due to low mortality rates in such studies; still, based on limited data, mortality was lower in the lifestyle intervention arms than in the control arms, although the difference was not significant.15 As the standard of care, lifestyle interventions are an important component of management for obesity and overweight, and they may help in achieving clinically meaningful weight-loss goals; however, some patients may need adjunctive therapy to lose weight or maintain weight loss.7,8
Bariatric Surgery
Bariatric surgery can be highly effective for weight loss in eligible patients, and it is recommended for patients with severe obesity, as discussed in the third article of this supplement.5,7 The results from a meta-analysis of 45 studies on the efficacy of bariatric surgery found that 1 year after surgery, patients who underwent any type of procedure showed superior percentages of excess weight loss vs controls, and this trend remained at 2 and 3 years after surgery for certain procedures.16 Further, results of an ongoing, prospective, controlled Swedish study assessing long-term (median, > 20 years) mortality outcomes associated with bariatric surgery in patients with obesity showed that bariatric surgery was associated with a significantly lower risk of mortality (P < .001) and a decreased risk of mortality due to CVD or cancers than was conventional obesity care.17
In November 2022, the American Society for Metabolic and Bariatric Surgery released a joint statement with the International Federation for the Surgery of Obesity and Metabolic Disorders that recommends bariatric surgery for individuals with a BMI of at least 35 kg/m2 regardless of weight-related complications, for patients with a BMI of at least 30 kg/m2 who have T2D, and for those with a BMI of 30 kg/m2 to 34.9 kg/m2 who have not achieved “substantial and durable” weight loss or improvement in complications using nonsurgical methods.18 The statement notes that the definition of obesity via BMI thresholds does not apply similarly to all populations, and access to bariatric surgery should not be denied based on BMI criteria alone. At the time of writing, this statement has not been evaluated by authors of the 2016 AACE/ACE treatment guidelines, which state that bariatric surgery should only be considered for patients with a BMI of at least 40 kg/m2 or those with a BMI of at least 35 kg/m2 and at least 1 weight-related complication.7 Thus, in practice, bariatric surgery may not be an option for those with overweight or with obesity and a lower BMI. Various bariatric surgery procedures may also be associated with short- or long-term AEs, such as pain, development of hernias, wound infection, bleeding, ulcers, band slippage or erosion, gallstone formation, nausea and vomiting, strictures, malnutrition, dumping syndrome, need for revision surgeries, and gastrointestinal (GI) obstruction, leakage, or perforation.16,19 For the large proportion of patients with obesity and overweight who do not qualify for, or who are unwilling to consider, bariatric surgery, use of AOMs or other treatment options should be considered.
Pharmacotherapy
The AACE/ACE treatment guidelines for obesity suggest that patients with a BMI of at least 27 kg/m2 are eligible for weight management with AOMs.7 The guidelines also state that all FDA-approved AOMs, when combined with lifestyle intervention, produce greater and more sustained weight loss when compared with lifestyle intervention alone, and the degree of weight loss achieved with the addition of these medications is associated with greater health benefits.7 In November 2022, the American Gastroenterological Association (AGA) released new guidelines on pharmacologic treatment for adults with obesity to support decision-making by patients and health care providers (HCPs) about currently available AOMs.20 The panel considered the benefits and harms derived from a systematic review and meta-analysis of RCTs published through the end of 2021 on subcutaneous injection of semaglutide 2.4 mg (Wegovy®; Novo Nordisk) and liraglutide 3.0 mg (Saxenda®; Novo Nordisk), as well as the following oral agents: phentermine-topiramate extended release (ER) (Qsymia; VIVUS), naltrexone-bupropion ER (Contrave; Currax Pharmaceuticals), orlistat, phentermine, diethylpropion, and Gelesis100 superabsorbent hydrogel (Plenity; Gelesis).20-25 The AGA guidelines strongly recommend AOMs plus lifestyle intervention in patients with obesity or overweight with comorbidities who have not responded adequately to lifestyle intervention alone; further, they note that chronic use of AOMs is generally necessary.20 With the exceptions of orlistat (recommendation against) and Gelesis100 oral superabsorbent hydrogel (no recommendation due to a gap in knowledge), all treatments analyzed were recommended over lifestyle intervention alone in eligible patients. The authors affirmed that “the selection of the medication…should be based on the clinical profile and needs of the patient, including, but not limited to, comorbidities, patients’ preferences, costs, and access to the therapy.”20
A 2022 report from the Institute for Clinical and Economic Review (ICER) reviewed the efficacy and safety of 4 AOMs: semaglutide, liraglutide, phentermine-topiramate ER, and naltrexone-bupropion ER.26 Results showed that use of any of the AOMs in combination with lifestyle intervention produced significantly greater mean weight loss than did placebo with diet and exercise counseling at 1 year (P < .05). However, AOMs also were associated with higher discontinuation rates than was placebo.26 Furthermore, the USPSTF systematic review assessing the effectiveness of orlistat, naltrexone-bupropion, phentermine-topiramate, liraglutide, or oral lorcaserin (Belviq; Eisai) compared with control found that AOM groups lost more weight, were more likely to lose 5% of their weight, and generally maintained greater weight loss compared with control groups, although some studies had high dropout rates and short follow-up durations.14,27 Rates of serious AEs were generally similar between arms; however, AOMs were associated with more frequent drop-out and higher AE rates than was placebo treatment.14 However, this study did not review data published after March 2018, and the results do not reflect the withdrawal of lorcaserin from the US drug market in 2020.27
According to prescribing information for AOMs with indications for chronic weight management in patients with obesity or with overweight in the presence of at least 1 weight-related complication, GI AEs are the most reported; other common AEs include paresthesia, dizziness, dysgeusia, insomnia, dry mouth, headache, injection site reaction, fatigue, and, in patients with T2D, hypoglycemia.21-24,28 Despite potential AEs, the guidelines clearly recommend that AOMs be available for eligible patients.7,8
Barriers to Treatment
The currently recommended clinical treatment guidelines for obesity involve therapeutic options based on a foundation of lifestyle intervention (including behavioral modification, healthy meal plans, and increased physical activity) in addition to other modalities, including bariatric surgery and AOMs.7,8
However, effective obesity treatment can be hindered by patient and HCP perceptions of obesity and associated treatments, low insurance coverage, social determinants of health that impact accessibility, challenges with treatment adherence, and other factors.
Patient Perceptions
Barriers to effective screening and diagnosis of overweight and obesity may include patient feelings of discomfort or stigmatization in health care settings, prior negative experiences, or the belief that seeking help indicates personal failure.29 Although 65% of people with obesity surveyed as part of the Awareness, Care and Treatment In Obesity MaNagement (ACTION) study (NCT03223493) believed that obesity was a disease, 82% agreed that their weight loss was completely their responsibility, and 44% of those who did not seek help from HCPs for weight loss cited personal responsibility as a reason.30 This survey explored the perceptions of and potential barriers to obesity care based on responses from patients with obesity (n = 3008) and HCPs (n = 606) in the fall of 2015. Most people with obesity agreed that improved eating habits/calorie reduction and increased physical activity were “completely effective” for long-term weight management (80% and 81%, respectively); however, they perceived lower efficacy for bariatric surgery (40%) and AOMs (27%).30 According to the authors, these perceptions may impact uptake of proven effective weight management interventions, and HCPs have an opportunity to facilitate discussions about obesity with patients who believe that they are completely responsible for their weight loss. However, self-reported data (such as BMI measures) gleaned from the ACTION surveys may prove unreliable, and selection of respondents may have been skewed due to the survey response rate (10%-20%) and average length of time needed to complete the survey (> 37 minutes).30
Patients are often receptive to HCP counseling on weight management, and they may be more likely to engage in a weight-loss attempt or to seriously consider bariatric surgery if their HCP advises them to do so.29,31,32 However, not only may patients perceive different levels of effectiveness for various obesity interventions, but patient characteristics are associated with differences in openness to or consideration of discussion about obesity and treatments with HCPs. Results from 1 survey of adult patients with overweight and obesity found that compared with White respondents, those from other racial/ethnic backgrounds were more likely to want weight-related discussions with their HCPs.33 Results from a different survey of adult patients with a BMI greater than 35 kg/m2 showed that compared with women and White patients, men and African American patients were less likely to seriously consider undergoing bariatric surgery.32
Provider Perceptions
Most HCPs surveyed in the ACTION study agreed that dietary (81%) and physical activity (79%) improvements were “completely effective” for weight loss, yet fewer believed the same of bariatric surgery (62%) or AOMs (30%).30 A separate online survey examined obstacles to obesity management perceived by HCPs in an academic Michigan health system.34 Although most reported addressing BMI during clinic visits, only about half of the providers had discussed weight loss during over 50% of visits with patients with obesity. Among treatments offered, counseling on lifestyle changes was almost universally reported, and discussion about bariatric surgery or AOMs with eligible patients was lower. Reported barriers to offering bariatric surgery included concerns about AEs and surgical risk, lack of either comfort with initiating discussion of surgery or experience with timing for offering surgery, and limited knowledge about bariatric surgery or long-term outcomes. Over 80% of respondents did not offer AOMs to patients with obesity; reported barriers to prescribing AOMs included lack of experience with or awareness of medications, concerns about AEs, high costs or issues with prior authorizations, and perceived lack of efficacy. Limitations of this study included a small sample size (N = 111), use of a non-validated questionnaire, and a survey population of only primary care providers and endocrinologists.34
In both surveys, HCPs most often cited lack of time during an appointment as an obstacle to initiating discussions of weight loss with patients, and some providers also reported discomfort with broaching the subject.30,34 HCPs indicated that they do not provide guideline-recommended counseling on weight management because of inadequate training and counseling skills, lack of time or clinic space and resources, low confidence in efficacy of obesity counseling, and reimbursement challenges.29 Further, authors of a survey study examining weight-related care experiences in adult patients with overweight and obesity posited that providers’ lack of comfort with or biases against bariatric surgery or AOM use may have contributed to results showing that most eligible patients received no information about bariatric surgery or AOM treatment.33 Indeed, an analysis of 2009-2015 data from 8 different health networks showed that 8.3% of prescribers wrote approximately 90% of all filled AOM prescriptions; the authors noted that this trend may have resulted from such prescribing barriers as provider biases, concerns about safety and efficacy, and a lack of uniform and consistent treatment coverage.35
Treatment Coverage
In 2002, the Internal Revenue Service issued a ruling including expenses for obesity treatment as deductible medical expenses.36 That same year, the Social Security Administration determined that obesity was a valid medical source of impairment for disability claims, further supporting the financial needs of patients with obesity and overweight.36 In 2006, the Centers for Medicaid & Medicare Services (CMS) began providing coverage for bariatric surgery under Medicare, removing a significant barrier for patients with obesity who were seeking surgical care.36 However, health plans for federal employers still could exclude obesity care by characterizing obesity as a lifestyle or cosmetic condition.36 This continued until 2014, when the federal Office of Personnel Management issued guidance to health plans that prohibited the practice for federal employers, acknowledging that AOMs can assist adults with obesity who “do not achieve weight loss goals through diet and exercise alone.”36,37 States were encouraged to follow suit in 2015, when the National Conference of Insurance Legislators released a resolution urging state legislators, health departments, and other state agencies and institutions to prioritize the prevention and treatment of obesity, including “coverage of the full range of obesity treatments, particularly new innovative measures such as pharmacotherapy and bariatric surgery” to decrease the direct and indirect negative effects of obesity on patient health and quality of life and the US economy.36,38
Current legislation is pending to expand protections for persons with obesity and overweight and to encourage treatment availability and access. The Treat and Reduce Obesity Act of 2021, proposed in the US Senate in March 2021, would update Medicare’s rules to expand the list of HCPs (eg, physicians, physician assistants, nurse practitioners, registered dietitians) qualified to provide covered intensive behavioral treatment and to expand Medicare Part D coverage to include AOMs for those with obesity or overweight and 1 or more comorbidities.39
However, current insurance coverage often does not align with obesity treatment guidelines. Providers using evidence-based clinical treatment guidelines to treat patients with obesity face the challenge that private and public health insurers often do not cover certain treatments, as noted in a recent consensus statement from an international expert panel.40,41 Only 13% of employed people with obesity in 1 study reported that their employers offered “insurance coverage for the medical treatment of obesity,” although the study did not provide details about coverage for specific obesity treatment modalities.30
Coverage of Lifestyle Intervention
Lifestyle intervention forms the foundation of obesity treatment, and long-term counseling can improve long-term weight loss and maintenance.7,8,29 However, coverage of behavioral counseling for weight loss can vary based on a patient’s insurance coverage and specific diagnoses.
As of November 29, 2011, CMS offers insurance coverage for intensive behavioral therapy for patients with a BMI of at least 30 kg/m2 to promote prevention and early detection of associated illness and disability. Intensive behavioral therapy includes obesity screening, dietary assessment, and intensive behavioral counseling and therapy to promote long-term weight loss through high-intensity diet and physical activity interventions.42
In 2018, the USPSTF recommended that “intensive, multicomponent behavioral interventions (ie, behavior-based weight loss and weight loss maintenance interventions)” be offered to or referred for adults with a BMI of 30 kg/m2.43 This recommendation was given a grade “B” rating, indicating moderate net benefit. The Patient Protection and Affordable Care Act (ACA) requires group health plans and health insurance issuers that offer group or individual health insurance coverage to cover and impose no cost sharing requirements on services and treatments related to USPSTF recommendations that receive a grade “A” or “B” rating.44 However, Medicare currently covers behavioral therapy only in the primary care setting, and nutritional counseling is only covered for patients with certain comorbidities (eg, diabetes, kidney disease); in both cases, Medicare may not cover the frequency or specific services an HCP recommends, and patients may still have out-of-pocket costs.40,45,46
Coverage of Bariatric Surgery
Coverage of bariatric surgery for qualifying individuals is generally high, although only certain patients with obesity are eligible. Since February 21, 2006, CMS offers coverage for several bariatric surgery procedures for Medicare beneficiaries with a BMI of at least 35 kg/m2 and at least 1 weight-related complication who have been unsuccessful with other medical treatments for obesity. However, CMS will not cover bariatric surgery to treat obesity in patients without associated comorbidities.47
Private insurers often provide conditional coverage for bariatric surgery, as well. In a survey of bariatric surgery coverage policies from the 64 private insurance companies with the highest US market shares using policies that were updated between 2017 to 2018 or collected by phone from company representatives, 61 companies (95%) had defined medical policies for bariatric surgery coverage, whereas the 3 remaining companies did not provide coverage. Among private payers, coverage varied for different procedure types. Further, 56 companies (92%) required that patients meet a criterion of a BMI of at least 40 kg/m2 or of at least 35 kg/m2 with at least 1 weight-related complication, and 53 companies (87%) required completion of a supervised medical weight management program. A minority of insurers required an obesity diagnosis 1 to 5 years before surgery, and some insurers required the presence of particular comorbidities (eg, T2D, hypertension, obstructive sleep apnea, coronary artery disease). All policies also required prior authorization and covered very few or no out-of-network costs.48
Coverage of Pharmacotherapy
As of 2021, only Virginia and New Mexico mandated AOM coverage for health benefits under the ACA.40 In 2014, considering the Federal Employees Health Benefits Program’s widespread exclusion of medications for weight management, the US Office of Personnel Management published a supplemental guidance stating that federal coverage of AOMs cannot be denied based on the notion that obesity is a “lifestyle” condition or that obesity treatment is “cosmetic.”37 In addition, it emphasized that there is no prohibition of carriers extending coverage to include AOMs. However, insurance coverage of AOMs remains highly variable.
Medicare excludes medications for weight management, including for noncosmetic weight loss, from the definition of a Part D drug. Medicare Part D plans may choose to provide enhanced alternative coverage plans that cover AOMs as a supplemental benefit; however, beneficiaries who opt for a Part D plan offering supplemental benefits must pay the full premium, as Medicare does not subsidize these benefits. An in-depth investigation into the expenditures on, prescriptions for, and insurance coverage of 9 AOMs (phentermine, diethylpropion, benzphetamine, phendimetrazine, orlistat, lorcaserin, phentermine-topiramate ER, liraglutide, and naltrexone-bupropion ER) from the US Government Accountability Office (GAO) found that only 555 claims from 209 beneficiaries were reimbursed for AOMs in 2017 under this enhanced alternative coverage. Further, coverage of AOMs is optional for state Medicaid and Medicaid managed care plans. The GAO report was based on a review of literature from January 2012 through January 2019, including analyses of federal agency data and interviews with stakeholders from 3 medical associations and 5 advocacy groups. It showed that among states that reimbursed for AOMs in 2017, approximately half covered fewer than 100 claims; further, more than half of all AOM reimbursements were for generic phentermine, which is approved only for short-term use (generally, ≤ 12 weeks).49
According to the GAO report’s assessment of private employer-sponsored and individually purchased health plans, a substantial proportion of plans provided coverage for AOMs in principle, but many had requirements (prior authorization, determination of medical necessity) that inevitably reduced access. Some plans offered no coverage at all, whereas others covered AOMs as a nonformulary option after a patient tried other treatment options (eg, behavioral intervention). Overall, older AOMs were more likely to be covered, and newer drugs were more likely to be associated with prior authorizations or higher co-pays.49
The GAO also reported that between 2012 and 2016, more than two-thirds (68%) of all money spent on AOMs in the United States involved out-of-pocket payments made by patients (Figure).49 These results may indicate the extent to which private and public insurers may not meet the coverage needs of patients. Implications of this report were limited by the analysis of only 9 AOMs approved by the FDA as of June 2019 for short- or long-term treatment of obesity and the imprecise estimates of payments. In addition, reimbursement data included coverage by Medicaid managed care organizations that could provide coverage for AOMs not covered by state plans; Medicaid and Medicare plans that covered an AOM but had no enrolled beneficiaries fill such a prescription were not captured.49
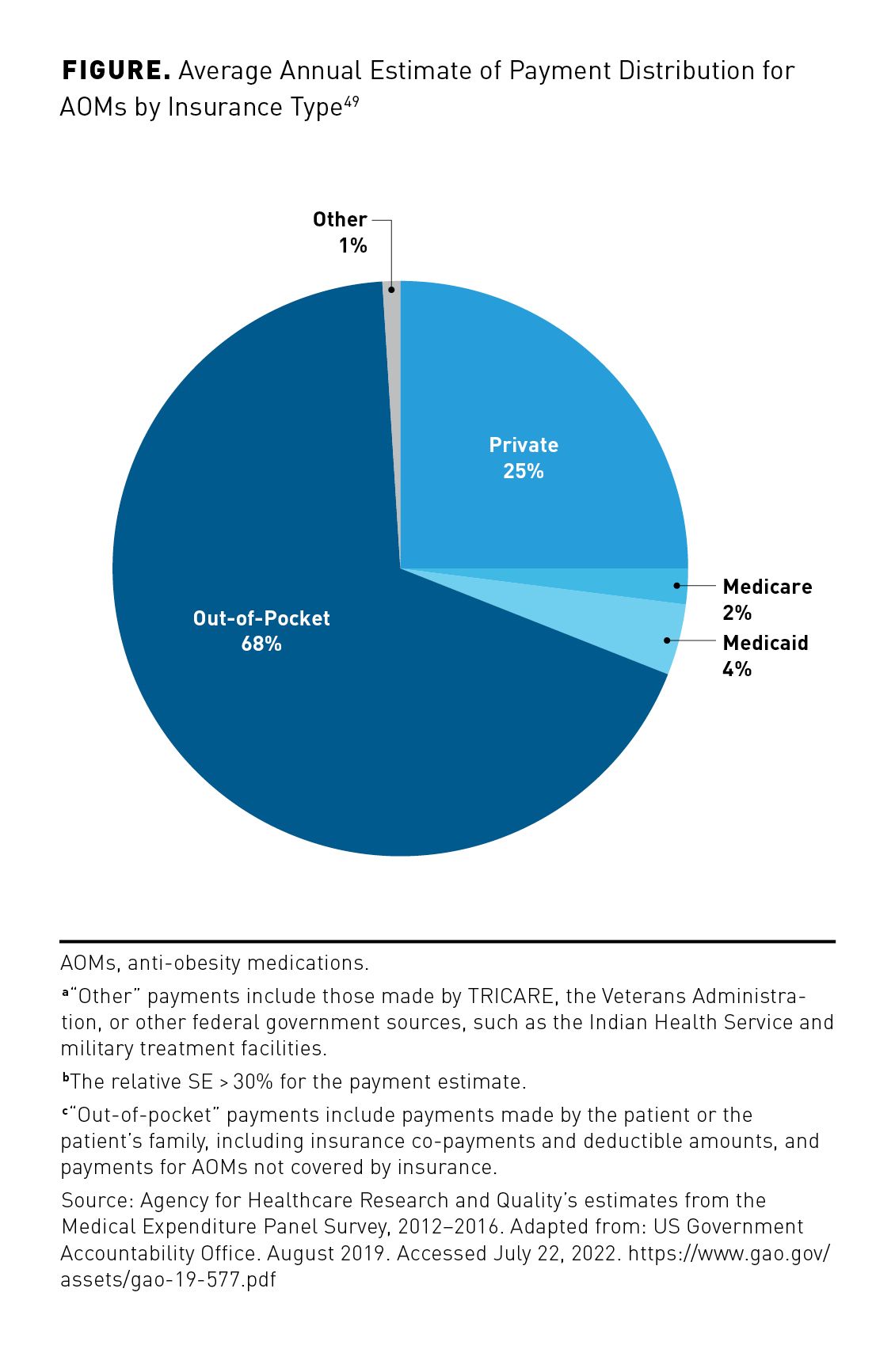
Treatment Adherence
Adherence to weight management interventions may be difficult for patients with overweight or obesity. A systematic review and meta-analysis of 27 RCTs and observational studies (2004-2015) on lifestyle intervention adherence found that the overall adherence rate was 60.5%.50
Sustaining lifestyle or behavioral changes increases the chance of weight loss. However, adherence to nutritional and physical activity regimens can be difficult to maintain and may often wane after the initial period of weight loss.9 This trend may result from the perception that the physical and cognitive efforts required during initial weight loss feel less worthwhile when maintaining achieved weight loss. Additionally, achievement of weight loss outcomes may vary, and weight loss early in the intervention can be predictive of long-term success. These factors serve as elements of a larger physiologic feedback loop, wherein weight loss induces biologic changes that lead to increased appetite and disproportionately decreased energy expenditure.9 Additionally, metabolic alterations enhance the body’s use and storage of nutrients and encourage the use of carbohydrates, rather than fat, for energy production. These adaptive responses typically do not reset when a reduced body weight is achieved, and they may even become exacerbated over time, promoting weight regain. Thus, although lifestyle intervention is the foundation of obesity treatment, psychological and physiologic barriers may be obstacles to successful and sustained weight loss via lifestyle intervention alone.9,13
Recommended postoperative care for patients undergoing bariatric surgery includes regular follow-up appointments to monitor metabolism and nutrition, consistent physical activity, and evaluation of vitamin levels and need for supplementation in patients at risk for deficiencies.51,52 Increased adherence to these measures is associated with improved outcomes. However, dietary and physical adherence after surgery is often low (< 50%), and adherence to follow-up visits drops substantially over time.52
For patients who receive a prescription for an AOM, ongoing use—required to maintain treatment benefits—can be challenged by the poor rates of medication adherence.53-55 More than half of patients prescribed long-term AOMs may discontinue or switch treatment within 6 months of initiation (often within the first month), although risk of discontinuation varies with use of different AOMs.54,55
Social Determinants of Health as Obstacles to Treatment
Social determinants of health (SDOH) include a network of interrelated issues that may impact a person’s health, including socioeconomic, geographic, occupational, educational, and environmental factors. SDOH can impact weight control through conditions that influence related behaviors; these include income and debt, employment opportunities and health insurance, access to education and nutritious food choices, cost of housing, and characteristics of the neighborhood or locality in which a person resides. Populations negatively impacted by SDOH may face substantial obstacles to achieving weight loss due to associated health inequities.56,57
Sociodemographic criteria may impact the risk of experiencing obesity and patient access to obesity treatments. The results of a Centers for Disease Control and Prevention analysis of National Health and Nutrition Examination Survey data from 2011 to 2014 found that adults in lower income brackets generally had a greater risk of obesity (BMI ≥ 30 kg/m2) compared with those in the highest income bracket, as did adults with lower educational levels compared with those with higher educational levels.58 HCPs who treat patients with obesity should consider that these groups may be at greater risk for obesity and may have less access to or understanding of health care due to social determinants.57-59 Indeed, patients with lower educational attainment and those living below the poverty level may have lower levels of health literacy, defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”57,59
Clinical guidelines represent what may be considered the ideal for obesity treatment; however, patients negatively impacted by SDOH may not have the capacity or resources necessary to follow such comprehensive treatment plans. Thus, providers should weigh patient preferences and practical considerations to individualize obesity treatment. For example, financial security may influence access to resources that promote healthy weight (eg, gyms and trainers, nutritious foods, and health insurance coverage).7,57,60 People with higher family incomes are more often insured, and they tend to have higher rates of private health insurance coverage.60 Results from the previously mentioned meta-analysis of studies on adherence to lifestyle intervention found that higher socioeconomic status was associated with higher adherence.50 Further, according to data from a separate international meta-analysis of 9 studies involving nearly 65 million eligible adult patients, those with private insurance were 2.5 times more likely to receive bariatric surgery than were those with nonprivate insurance.61 Finally, in a study analyzing National Ambulatory Medical Care Survey data (2011-2016) on AOM mentions, individuals with private insurance were significantly more likely to have an AOM mention than were those with public insurance (eg, Medicare, Medicaid), other coverage (including workers’ compensation, no charge, or charity), or missing insurance information or self-pay patients, regardless of patient obesity status (P < .0001).62 Authors of the study posited that this disparity may be due to low public health insurance coverage of these drugs, although they noted that the study design may have led to an underestimation of AOM mentions (ie, consideration of only orlistat, phentermine, diethylpropion, benzphetamine, phendimetrazine, liraglutide, lorcaserin, naltrexone-bupropion ER, or phentermine-topiramate ER).62
Payers are encouraged to examine procedural requirements for coverage of obesity treatment. For example, requiring weekly personalized weight management sessions may create unnecessary barriers to care (eg, cost-prohibitive treatment, patient lack of transportation or inability to get time off work). Payers that will not cover AOMs or bariatric surgery for patients unable to participate in an intensive lifestyle intervention should consider the possible burden of such restrictions on socially or economically disadvantaged patients.
Managed Care Considerations
In the second article of this supplement, Danielle C. Massie, PharmD, and colleagues discuss the clinical burden of obesity, including how many common diseases (eg, T2D, CVD, osteoarthritis, asthma, depression, certain cancers) may be associated with or exacerbated by excess adiposity.4 In addition, the risk for weight-related complications can increase in proportion with duration of sustained obesity, demonstrating the profound health consequences that may result from obesity treatment barriers. Investigators on the Coronary Artery Risk Development in Young Adults (CARDIA) multicenter longitudinal cohort study (NCT00005130) analyzed the risk of CVD based on years of obesity or overweight (≥ 25 kg/m2) in 4061 eligible Black and White adult participants.63 The results indicated that greater degree and longer duration of obesity predicted the risk of CVD development in a dose-dependent manner.63 For example, sustaining a BMI of 30 kg/m2 for 8 years (equivalent to 50 excess BMI-years) carried a 20% increased risk of CVD, 25% increased risk of coronary heart disease, and 45% increased risk of heart failure.63 Similarly, investigators in an analysis of pooled data from 20,746 participants in 3 long-term birth cohort studies in the United Kingdom assessed the effects of obesity duration over the life course on cardiometabolic disease risk factors in mid-adulthood.64 Serial BMI measurements (total, 5-6) were recorded for each participant from age 10 to 40 years; this was followed by a single biomedical analysis (measurements of blood pressure, lipids, and hemoglobin A1c [HbA1c]) when participants had reached their 40s or 50s. Longer obesity duration was associated with higher HbA1C levels, increased systolic and diastolic blood pressure measurements, increased risk of hypertension, and lower high-density lipoprotein cholesterol levels compared with those of patients who never had obesity (P < .001 for all trends).64
Investigators on another study analyzed the risk of developing T2D associated with cumulative duration of overweight and obesity over nearly 2.5 million person-years of follow-up in 2 cohorts of women who were registered nurses in the United States.65 Results specific to obesity showed that for every 2 years of sustained obesity, T2D risk increased by 14%. Further, among women who had obesity for more than 9 years, the risk of developing T2D was nearly 5 times greater than seen among women who never had obesity, including those with normal or overweight (risk ratio, 4.82; 95% CI, 4.31-5.38).65 Finally, the results of a multicenter longitudinal study examined the relationship between sustained overweight or obesity duration and development of obesity-related cancer (cancer of the breast [postmenopausal], colon, rectum, liver, gallbladder, pancreas, endometrium, ovary, kidney, or thyroid) in 73,913 postmenopausal women who were cancer-free at baseline.66 Results specific to obesity showed that for every 10 years of obesity, there was a 10% increased risk of developing an obesity-related cancer.
Reported health consequences should be interpreted in the context of study limitations (eg results may not be generalizable due to limited study populations, presence of confounding due to observational study design or differences in height and weight measurement protocols, reliance on self-reports for some data, and risk of missing data inherent in such longitudinal studies).63-66 Overall, however, the results support that the health consequences of obesity compounded over time. Therefore, timely access to obesity treatment may prevent complications in the long term, and patients may benefit from achieving weight loss regardless of how long they live with obesity.
Quality Measures in Obesity
CMS has tied quality measures to fee-for-service payments to incentivize quality of health outcomes over quantity of procedures. A search of quality measure databases and professional societies conducted in October 2016 and in January 2017 revealed 11 quality measures focused on obesity or BMI in US adults.67 Only 2 of 11 measures were actively used within CMS programs at the time of analysis. The measure Adult BMI Assessment calculated the percentage of patients aged 18 to 74 years who had an outpatient visit and whose BMI was documented in the previous 2 years; however, this measure was retired in 2020.67,68 The measure Preventive Care and Screening: BMI Screening and Follow-Up focuses on percentage of adult patients with BMI documentation during the current encounter or within the previous 12 months and with a documented follow-up plan when BMI is outside of the normal weight parameters.69 For this measure, the follow-up plan may include documentation of education, referral to an HCP for lifestyle or behavioral therapy, pharmacologic interventions, dietary supplements, and physical activity or nutrition counseling.69 Thus, health care systems that encourage HCPs to implement appropriate treatment may be less likely to receive reduced CMS reimbursement.
Additional quality measures in obesity treatment have been investigated. A recent observational study investigated the performance of obesity quality measures using retrospective quantitative data; results showed that the measure Documentation of Obesity Diagnosis—the percentage of adult patients (age, 18-79 years) with at least 1 ambulatory visit during the measurement year, BMI of at least 30 kg/m2, and a documented obesity diagnosis—was feasible (performance rates at different sites, 9.8%-35.0%) and reliable (reliability score [scale, 0.0-1.0], 0.996). Although validity of this measure was supported, further testing is needed to improve content validity and scalability of measures focused on improving and documenting obesity care.70
Rates of obesity diagnoses in eligible patients attending normal clinic visits could be improved. In the ACTION study, only 55% of patients with obesity who had discussed their weight with their HCP over the previous 5 years reported receiving a diagnosis of obesity.30 In an analysis of 2015 electronic health records in patients 20 years or older at the Cleveland Clinic, only 48% of patients with a BMI of at least 30 mg/m2 (n = 134,488) had a documented diagnosis of obesity.71
Receiving a formal diagnosis of obesity is associated with increased odds of having an obesity management plan or losing weight.29 Thus, documented, confirmed obesity diagnoses can facilitate discussion between HCPs and patients about obesity, patient engagement in weight management interventions, and patient weight loss.70 A quality measure that encourages documentation of obesity diagnoses in patient claims or electronic health records may promote positive outcomes in individuals with obesity, and it also can provide information for population identification and planning of larger health initiatives.70
Potential Health Care Costs Savings with Obesity Treatments
Obesity and overweight are risk factors for several chronic diseases.72 Estimated direct medical costs of treating these conditions total in the hundreds of billions of dollars annually; when considering the indirect costs due to lost economic productivity, the total cost of chronic diseases due to obesity increases into the trillions of dollars.72 However, clinically significant weight loss (5%-10% of body weight) can improve clinical markers of weight-related conditions and lower patient risk of certain comorbidities.7,9 Therefore, obesity treatments that can help patients achieve therapeutic weight loss may, by extension, reduce health care costs.
Nonsurgical weight management treatments include lifestyle intervention and AOMs.7,73 These may be associated with significant short-term (2 years) health care cost savings (P < .05) for patients achieving sustained clinically meaningful weight loss, according to the results of a retrospective analysis of electronic medical record data in over 15,000 patients with obesity between 2012 and 2018.7,9,73
A study modeling Medicare savings calculated that moderate (50%) or extensive (67%) expansion of coverage for lifestyle intervention with or without AOM use would increase treatment utilization and result in billions of dollars in savings over 10 years.74 A separate study modeling the economic effects of potential patient weight loss associated with 100% AOM uptake on Medicare, Medicaid, and disability payments estimated that despite increased retirement payouts due to increased survival, the US government would save billions of dollars over 10 years and, potentially, hundreds of billions of dollars over 75 years.75 When estimating health care costs across the United States over the long term, widespread coverage of lifestyle intervention and AOMs is modeled to result in substantial cost savings.
Bariatric surgery is highly effective for weight loss in patients with severe obesity, and it may be associated with long-term cost savings.7,76 For example, the results of a systematic review and meta-analysis of pooled data from bariatric surgery cost-utility research published through July 2019 in high-income countries (including the United States) found that despite high variability of study results, bariatric surgery can be cost-effective vs usual care (ie, pharmacotherapy and/or lifestyle intervention) over 10 years and over a lifetime horizon.76 Unfortunately, there is a dearth of similar studies modeling the long-term cost impact of bariatric surgery on the US health care system, specifically. Surgical weight management procedures can be associated with substantial upfront costs in the United States, including the high inpatient costs associated with bariatric surgeries, and limited US data suggest that this modality may not produce significant long-term direct health care cost savings over 6 to 10 years.77-79 However, these results fail to consider the potential effects of bariatric surgery on indirect costs associated with obesity (eg, premature morbidity and mortality, income and productivity losses, higher disability payments)79-81 or on lifetime health care costs for patients and payers.
Semaglutide 2.4 mg for Obesity—Clinical Evidence and Place in Therapy
The third article in this supplement discusses the broader treatment landscape of AOMs and their guideline-recommended place in therapy. There are currently 5 AOMs FDA-approved for long-term treatment of obesity. The newest is semaglutide 2.4 mg.5
Semaglutide is a GLP-1 receptor agonist that promotes weight management by limiting energy intake, increasing satiety, and reducing hunger.24,82 In 2021, semaglutide 2.4 mg delivered via subcutaneous injection received FDA approval as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adult patients with an initial BMI of at least 30 kg/m2 or at least 27 kg/m2 and in the presence of 1 or more weight-related complications (eg, hypertension, T2D, or dyslipidemia).24,83 Efficacy and safety results cited in the prescribing information come from 4 randomized phase 3a trials—STEP 1 (NCT03548935), STEP 2 (NCT03552757), STEP 3 (NCT03611582), and STEP 4 (NCT03548987)—in which patients with overweight or obesity received semaglutide 2.4 mg plus behavioral intervention over 68 weeks.24,82 The study populations for STEPs 1, 3, and 4 included either patients with obesity (BMI ≥ 30 kg/m2) or patients with overweight (BMI ≥ 27 kg/m2) and at least 1 non-T2D weight-related complication, whereas the study population for STEP 2 consisted of patients with a BMI of at least 27 kg/m2 and T2D.82
In all 4 trials, mean weight loss from baseline was significantly superior to that achieved by patients in the control arms (Table).84-87 Furthermore, in STEPs 1, 2, and 3, patients treated with semaglutide were significantly more likely to achieve at least 5% weight loss from baseline than were control patients (all P < .001; STEP 4 did not assess statistical significance of this measure).84-86
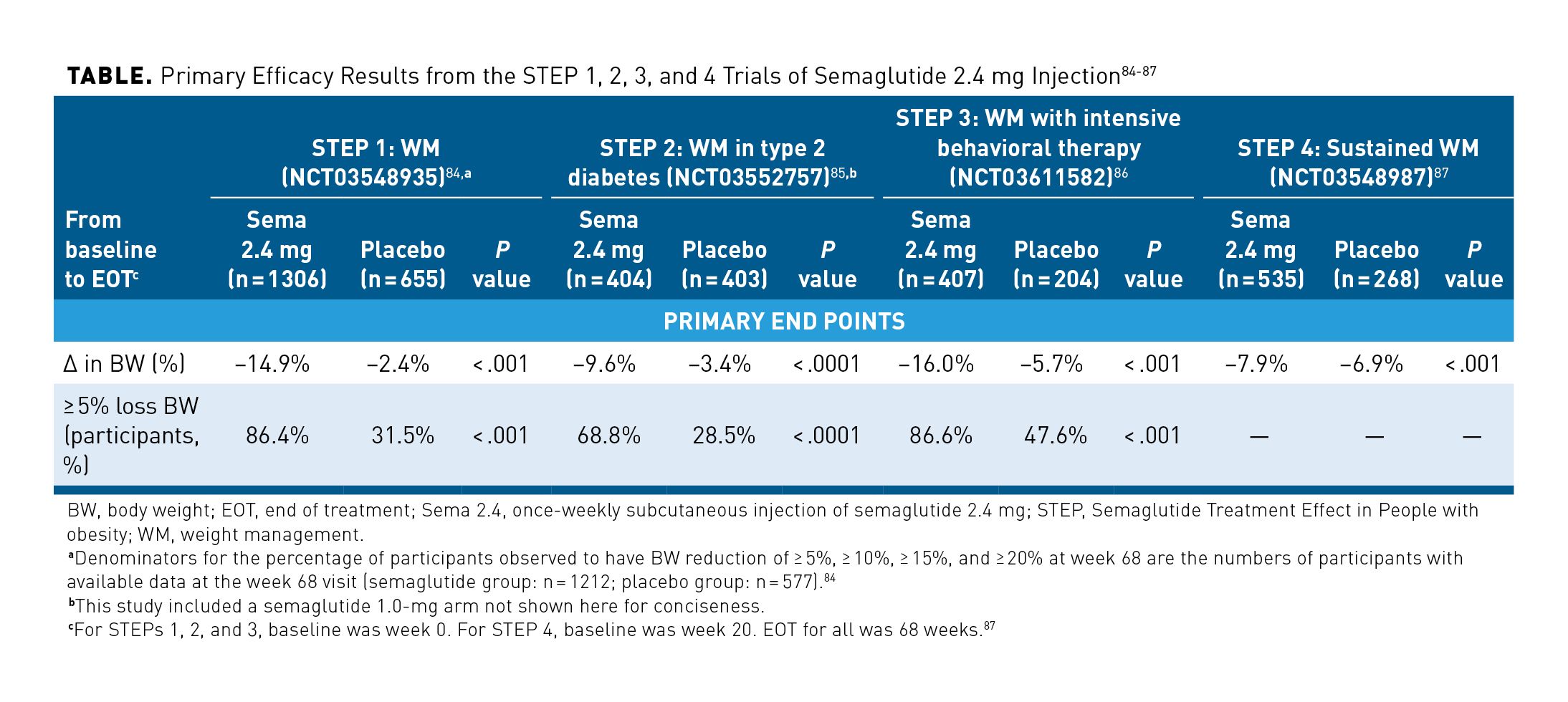
Thus, semaglutide 2.4 mg showed clinically meaningful reductions in weight.7 Reduced body weight was achieved with semaglutide 2.4 mg, regardless of patient age, sex, race, ethnicity, baseline body weight, or renal function.24 Further, because the trials analyzed AOM use plus lifestyle intervention, the results support the utility of this treatment option for patients who do not achieve meaningful weight loss on lifestyle intervention alone and who are unwilling or unable to undergo bariatric surgery.82 In the fourth article of this supplement, Janine Kyrillos, MD, explores results from the STEP trials in further detail.6
Evidence on the place for semaglutide 2.4 mg in therapy is evolving rapidly. In its 2022 guidance, the AGA states that “given the magnitude of net benefit, semaglutide 2.4 mg may be prioritized over other approved AOMs for the long-term treatment of obesity for most patients.” The panel also notes that uncertainty and variability in patient values and preferences on benefits and harms of treatment led to this recommendation being conditional.20
The previously mentioned ICER report analyzed the efficacy, safety, and cost-effectiveness of lifestyle intervention plus semaglutide 2.4 mg, liraglutide 3.0 mg, phentermine-topiramate ER, or naltrexone-bupropion ER. Use of semaglutide and phentermine-topiramate ER was associated with greater weight loss than was treatment with liraglutide or naltrexone-bupropion ER, and semaglutide therapy appeared to be associated with lower rates of discontinuation due to AEs than were the other drugs. In addition, the combination of semaglutide and lifestyle intervention was more effective, but more costly, than both phentermine-topiramate ER and naltrexone-bupropion ER. To meet typical cost-effectiveness thresholds, pricing of semaglutide would require considerable wholesale acquisition cost discounts; however, semaglutide has shown higher efficacy, a lower AE burden, and greater cost-effectiveness than has liraglutide.26
A separate study modeled the cost-effectiveness of semaglutide 2.4 mg vs other long-term use AOMs (ie, liraglutide 3.0 mg, phentermine-topiramate ER, naltrexone-bupropion ER) as an adjunct to lifestyle intervention in patients with overweight and obesity from a US third-party payer perspective. At a willingness-to-pay threshold of $150,000 per quality-adjusted life-year gained over a 30-year horizon, semaglutide was deemed to be more cost-effective than no treatment, lifestyle intervention alone, and use of other long-term AOMs. Model parameters were often based on trial data, and that may have limited generalizability of results to the real world, including the course of the disease and the patient population with obesity. Further, lifestyle intervention was modeled after individual counseling provided in the semaglutide STEP 1 trial to support calorie reduction and increased physical activity, which may not represent real-world lifestyle intervention. Finally, treatment efficacy was based upon naïve comparisons of respective pivotal trial data, as indirect or direct head-to-head comparisons of treatments were not available at the time of study completion.88
Conclusions
Social and practical barriers, patient and HCP biases, and health care affordability challenge implementation of widespread obesity treatment. However, views of obesity are changing. Since the official recognition of obesity as a chronic disease in 2013, implementation of the ACA has increased coverage for some obesity treatments. Furthermore, there is increasing evidence and awareness that certain treatment approaches may be more appropriate than others for individual patients. Lifestyle intervention is the foundation of effective weight management initiatives; alone, however, it may not result in successful weight loss or maintenance for a substantial proportion of people with excess adiposity. The addition of bariatric surgery or AOM use may aid in clinically meaningful weight loss that improves health and well-being. Future research should focus on the long-term clinical and economic outcomes associated with use of different interventions and the effects of providing access to and coverage of the full range of obesity treatments for eligible patients. Such research will inform treatment and coverage decisions at the population level, as obesity is a chronic disease that will require long-term individualized treatment. Despite being recommended by obesity treatment guidelines and showing the potential for long-term cost-effectiveness based on current evidence, AOMs—now including semaglutide 2.4 mg—largely are not covered by either public or private insurers. From a managed care perspective, successful treatment of obesity to improve health outcomes and reduce health care costs must take advantage of all available evidence-based modalities for achieving weight loss.
Acknowledgements
This peer-reviewed supplement was funded by Novo Nordisk Inc. The authors acknowledge the professional medical writing support from Clinical Communications, a division of MJH Life Sciences®, Cranbury, NJ, which received funding support from Novo Nordisk Inc, Plainsboro, NJ. Novo Nordisk Inc. provided scientific and medical accuracy review of this publication.
Author Affiliations: University of Pennsylvania (AA), Philadelphia, PA; Stony Brook University Hospital (MK), Stony Brook, NY; Moda Health (DCM), Portland, OR.
Funding Source: This supplement was supported by Novo Nordisk.
Author Disclosures: Dr Amaro reports serving as a paid consultant or paid advisory board member for Novo Nordisk and has received grants from Altimmune and Eli Lilly. Dr Kaplan has received speaker honoraria from Novo Nordisk. Dr Massie reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (AA, MK, DCM); analysis and interpretation of data (AA); drafting of the manuscript (MK, DCM); and critical revision of the manuscript for important intellectual content (AA, MK, DCM).
Address Correspondence to: Michael Kaplan, DO, Long Island Weight Loss Institute, 329 East Main Street, Suite 9, Smithtown, NY 11789. Email: drkaplan@LIWLI.com
References
- American Medical Association House of Delegates, 2013. Recognition of obesity as a disease. Resolution 420 (A-13). National Public Radio. May 16, 2013. Accessed October 5, 2022. https://media.npr.org/documents/2013/jun/ama-resolution-obesity.pdf
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Lazarus E, Ortiz-Pujols S. Increasing clinical awareness of obesity as a serious, chronic, relapsing, and treatable disease. Am J Manag Care. 2022;28(suppl 15):S271-S278. doi:10.37765/ajmc.2022.89290
- Massie DC, Amaro A, Kaplan M. Patient well-being and the clinical and economic burdens associated with obesity in the United States. Am J Manag Care. 2022;28(suppl 15):S279-S287. doi:10.37765/ajmc.2022.89291
- Cornier MA. A review of current guidelines for the treatment of obesity. Am J Manag Care. 2022;28(suppl 15):S288-S296. doi:10.37765/ajmc.2022.89292
- Kyrillos J. Semaglutide 2.4-mg injection as a novel approach for chronic weight management. Am J Manag Care. 2022;28(suppl 15):S297-S306. doi:10.37765/ajmc.2022.89293
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi:10.4158/EP161365.GL
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25; pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
- MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring). 2015;23(1):7-15. doi:10.1002/oby.20967
- Jin X, Gibson AA, Gale J, et al. Does weight loss reduce the incidence of total knee and hip replacement for osteoarthritis?-A prospective cohort study among middle-aged and older adults with overweight or obesity. Int J Obes (Lond). 2021;45(8):1696-1704. doi:10.1038/s41366-021-00832-3
- Okoniewski W, Lu KD, Forno E. Weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann Am Thorac Soc. 2019;16(5):613-625. doi:10.1513/AnnalsATS.201810-651SR
- Ness-Jensen E, Hveem K, El-Serag H, Lagergren J. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2016;14(2):175-82.e1-3. doi:10.1016/j.cgh.2015.04.176
- Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415
- LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172-1191. doi:10.1001/jama.2018.7777
- Singh N, Stewart RAH, Benatar JR. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: a meta-analysis of randomised trials. BMJ Open. 2019;9(8):e029966. doi:10.1136/bmjopen-2019-029966
- Park CH, Nam SJ, Choi HS, et al. Comparative efficacy of bariatric surgery in the treatment of morbid obesity and diabetes mellitus: a systematic review and network meta-analysis. Obes Surg. 2019;29(7):2180-2190. doi:10.1007/s11695-019-03831-6
- Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med. 2020;383(16):1535-1543. doi:10.1056/NEJMoa2002449
- Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. Published online October 18, 2022. doi:10.1016/j.soard.2022.08.013
- Ma IT, Madura JA 2nd. Gastrointestinal complications after bariatric surgery. Gastroenterol Hepatol (NY). 2015;11(8):526-535.
- Grunvald E, Shah R, Hernaez R, et al; AGA Clinical Guidelines Committee. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022;163(5):1198-1225. doi:10.1053/j.gastro.2022.08.045
- Qsymia. Prescribing information. VIVUS Inc; 2022. Accessed July 22, 2022. https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf?v=0722&_ga=2.255472968.1715974929.
1658528852-1399778943.1658528852&_gac=1.250808754.1658528898.EAIaIQobChMIw9C6tsWN-QIVocmUCR0d3wBPEAAYASAAEgIQJfD_BwE - Contrave. Prescribing information. Nalpropion Pharmaceuticals LLC; 2021. Accessed July 22, 2022. https://www.contravehcp.com/wp-content/uploads/Contrave_PI_MedGuide.pdf
- Saxenda®. Prescribing information. Novo Nordisk; 2022. Accessed July 22, 2022. https://www.novo-pi.com/saxenda.pdf
- Wegovy®. Prescribing Information. Novo Nordisk; 2021. Accessed July 22, 2022. https://www.novo-pi.com/wegovy.pdf
- Plenity. Instructions for use. Gelesis; 2021. Accessed October 28, 2022. https://www.myplenity.com/siteassets/components/pdfs/acq_hcp_plenity-physician-ifu_march_2021.pdf
- Atlas SJ, Kim K, Beinfeld M, et al. Medications for Obesity Management: Effectiveness and Value; Evidence Report. Institute for Clinical and Economic Review. August 31, 2022. Accessed October 5, 2022. https://icer.org/assessment/obesity-management-2022/
- Belviq, Belviq XR (lorcaserin) by Eisai: drug safety communication - FDA requests withdrawal of weight-loss drug. FDA. February 13, 2020. Accessed October 21, 2022. https://www.fda.gov/safety/medical-product-safety-information/belviq-belviq-xr-lorcaserin-eisai-drug-safety-communication-fda-requests-withdrawal-weight-loss-drug
- Xenical. Prescribing information. CHEPLAPHARM Arzneimittel GmbH/H2-Pharma; 2017. Accessed July 22, 2022. https://xenical.com/pdf/PI_Xenical-brand_FINAL.PDF
- Kahan SI. Practical strategies for engaging individuals with obesity in primary care. Mayo Clin Proc. 2018;93(3):351-359. doi:10.1016/j.mayocp.2018.01.006
- Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obesity (Silver Spring). 2018;26(1):61-69. doi:10.1002/oby.22054
- Golden A, Dhurandhar NV, Jinnett K, et al. Insights and perceptions of obesity management in people with obesity: results of the national ACTION study. Poster presented at: Obesity Week 2016; October 31 to November 4, 2016; New Orleans, LA. Poster TP-3178. https://www.actionstudy.com/content/dam/novonordisk/actionstudy/resources/documents/7.%20Publications_OW%202016%20poster.pdf
- Wee CC, Huskey KW, Bolcic-Jankovic D, Colten ME, Davis RB, Hamel M. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med. 2014;29(1):68-75. doi:10.1007/s11606-013-2603-1
- Lewis KH, Gudzune KA, Fischer H, Yamamoto A, Young DR. Racial and ethnic minority patients report different weight-related care experiences than non-Hispanic Whites. Prev Med Rep. 2016;4:296-302. doi:10.1016/j.pmedr.2016.06.015
- Simon R, Lahiri SW. Provider practice habits and barriers to care in obesity management in a large multicenter health system. Endocr Pract. 2018;24(4):321-328. doi:10.4158/EP-2017-0221
- Saxon DR, Iwamoto SJ, Mettenbrink CJ, et al. Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009-2015. Obesity (Silver Spring). 2019;27(12):1975-1981. doi:10.1002/oby.22581
- Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin North Am. 2016;45(3):511-520. doi:10.1016/j.ecl.2016.04.004
- Supplemental guidance: management of obesity in adults. US Office of Personnel Management. March 20, 2014. Accessed July 22, 2022. https://www.opm.gov/healthcare-insurance/healthcare/carriers/2014/2014-04.pdf
- Resolution in support of efforts to reduce the incidence of obesity and chronic disease. National Conference of Insurance Legislators. July 29, 2015. Accessed October 4, 2022. http://ncoil.org/wp-content/uploads/2016/04/07232015ObesityResolution.pdf
- Treat and Reduce Obesity Act of 2021, S.596, 117th Congress. (2021-2022). Congress.gov. March 4, 2021. Accessed July 22, 2022. https://www.congress.gov/bill/117th-congress/senate-bill/596
- Waidmann TA, Waxman E, Pancini V, Gupta P, Tabb LP. Obesity across America: geographic variation in disease prevalence and treatment options. Urban Institute. February 17, 2022. Accessed July 22, 2022. https://www.urban.org/research/publication/obesity-across-america
- Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26(4):485-497. doi:10.1038/s41591-020-0803-x
- National coverage determination: intensive behavioral therapy for obesity. Centers for Medicare & Medicaid Services. Effective November 29, 2011. Accessed July 22, 2022. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=353
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(11):1163-1171. doi:10.1001/jama.2018.13022
- US Government. H.R. 3590. Patient Protection and Affordable Care Act, 42 U.S.C. § 18001. Congress.gov. 2010. Accessed June 4, 2021. https://www.congress.gov/bill/111th-congress/house-bill/3590
- Obesity behavioral therapy. Medicare.gov. Accessed July 22, 2022. https://www.medicare.gov/coverage/obesity-behavioral-therapy
- Medical nutrition therapy insurance coverage. Medicare.gov. Accessed July 22, 2022. https://www.medicare.gov/coverage/obesity-behavioral-therapy
- National coverage determination: bariatric surgery for treatment of co-morbid conditions related to morbid obesity. Centers for Medicare & Medicaid Services. Effective September 24, 2013. Accessed July 22, 2022. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=57&ncdver=5&DocID=100.1&kq=true&SearchType=Advanced&bc=EAAAAAgAAAAA&
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30(2):707-713. doi:10.1007/s11695-019-04243-2
- Obesity drugs: few adults used prescription drugs for weight loss and insurance coverage varied. US Government Accountability Office. August 2019. Accessed July 22, 2022. https://www.gao.gov/assets/gao-19-577.pdf
- Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547-1559. doi:10.2147/PPA.S103649
- Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175-247. doi:10.1016/j.soard.2019.10.025
- Hood MM, Corsica J, Bradley L, Wilson R, Chirinos DA, Vivo A. Managing severe obesity: understanding and improving treatment adherence in bariatric surgery. J Behav Med. 2016;39(6):1092-1103. doi:10.1007/s10865-016-9772-4
- Shettar V, Patel S, Kidambi S. Epidemiology of obesity and pharmacologic treatment options. Nutr Clin Pract. 2017;32(4):441-462. doi:10.1177/0884533617713189
- Ahmad NN, Robinson S, Kennedy-Martin T, Poon JL, Kan H. Clinical outcomes associated with anti-obesity medications in real-world practice: a systematic literature review. Obes Rev. 2021;22(11):e13326. doi:10.1111/obr.13326
- Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res Clin Pract. 2018;143:348-356. doi:10.1016/j.diabres.2018.07.017
- National Academies of Sciences, Engineering, and Medicine 2019. A health equity approach to obesity efforts: proceedings of a workshop in brief. National Academies Press. https://nap.nationalacademies.org/catalog/25496/a-health-equity-approach-to-obesity-efforts-proceedings-of-a
- About social determinants of health (SDOH). Centers for Disease Control and Prevention. March 10, 2021. Accessed July 22, 2022. https://www.cdc.gov/socialdeterminants/about.html
- Ogden CL, Fakhouri TH, Carroll MD, et al. Prevalence of obesity among adults, by household income and education - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2017;66(50):1369-1373. doi:10.15585/mmwr.mm6650a1
- Kutner M, Greenberg E, Jin Y, Paulse, C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). National Center for Education Statistics. 2006. Accessed July 22, 2022. https://nces.ed.gov/pubs2006/2006483.pdf
- Cohen RA, Martinez ME, Cha AE, Terlizzi EP. Health insurance coverage: early release of estimates from the National Health Interview Survey, January-June 2021. National Center for Health Statistics. November 2021. Accessed July 22, 2022. https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur202111.pdf
- Bhogal SK, Reddigan JI, Rotstein OD, et al. Inequity to the utilization of bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2015;25(5):888-899. doi:10.1007/s11695-015-1595-9
- Claridy MD, Czepiel KS, Bajaj SS, Stanford FC. Treatment of obesity: pharmacotherapy trends of office-based visits in the United States from 2011 to 2016. Mayo Clin Proc. 2021;96(12):2991-3000. doi:10.1016/j.mayocp.2021.07.021
- Reis JP, Allen N, Gunderson EP, et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring). 2015;23(4):879-885. doi:10.1002/oby.21023
- Norris T, Cole TJ, Bann D, et al. Duration of obesity exposure between ages 10 and 40 years and its relationship with cardiometabolic disease risk factors: a cohort study. PLoS Med. 2020;17(12):e1003387. doi:10.1371/journal.pmed.1003387
- Hu Y, Bhupathiraju SN, de Koning L, Hu FB. Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity (Silver Spring). 2014;22(10):2267-2273. doi:10.1002/oby.20851
- Arnold M, Jiang L, Stefanick ML, et al. Duration of adulthood overweight, obesity, and cancer risk in the women’s health initiative: a longitudinal study from the United States. PLoS Med. 2016;13(8):e1002081. doi:10.1371/journal.pmed.1002081
- Zvenyach T, Pickering MK. Health care quality: measuring obesity in performance frameworks. Obesity (Silver Spring). 2017;25(8):1305-1312. doi:10.1002/oby.21884
- 2021 Quality rating system measure technical specifications. Centers for Medicare & Medicaid Services. 2020. Accessed July 22, 2022. https://www.cms.gov/files/document/2021-qrs-measure-technical-specifications.pdf
- Preventive care and screening: body mass index (BMI) screening and follow-up plan. Centers for Medicare & Medicaid Services. November 2020. Accessed July 22, 2022. https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2021_Measure_128_MIPSCQM.pdf
- Sampsel S, Whiton K, Donckels E, et al. Assessing opportunities to advance quality measures in adult obesity. Am J Manag Care. 2021;27(12):562-567. doi:10.37765/ajmc.2021.88794
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open. 2017;7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Waters H, Graf M. America’s obesity crisis: the health and economic costs of excess weight. Milken Institute. October 2018. Accessed July 22, 2022. https://milkeninstitute.org/sites/default/files/reports-pdf/Mi-Americas-Obesity-Crisis-WEB_2.pdf
- Ding Y, Fan X, Blanchette CM, Smolarz BG, Weng W, Ramasamy A. Economic value of nonsurgical weight loss in adults with obesity. J Manag Care Spec Pharm. 2021;27(1):37-50. doi:10.18553/jmcp.2020.20036
- Chen F, Su W, Ramasamy A, et al. Ten-year Medicare budget impact of increased coverage for anti-obesity intervention. J Med Econ. 2019;22(10):1096-1104. doi:10.1080/13696998.2019.1652185
- Kabiri M, Sexton Ward A, Ramasamy A, et al. Simulating the fiscal impact of anti-obesity medications as an obesity reduction strategy. Inquiry. 2021;58:46958021990516. doi:10.1177/0046958021990516
- Noparatayaporn P, Thavorncharoensap M, Chaikledkaew U, Bagepally BS, Thakkinstian A. Incremental net monetary benefit of bariatric surgery: systematic review and meta-analysis of cost-effectiveness evidences. Obes Surg. 2021;31(7):3279-3290. doi:10.1007/s11695-021-05415-9
- Doble B, Wordsworth S, Rogers CA, Welbourn R, Byrne J, Blazeby JM; By-the-Band Trial Management Group. What are the real procedural costs of bariatric surgery? A systematic literature review of published cost analyses. Obes Surg. 2017;27(8):2179-2192. doi:10.1007/s11695-017-2749-8
- Weiner JP, Goodwin SM, Chang HY, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148(6):555-562. doi:10.1001/jamasurg.2013.1504
- Smith VA, Arterburn DE, Berkowitz TSZ, et al. Association between bariatric surgery and long-term health care expenditures among veterans with severe obesity. JAMA Surg. 2019;154(12):e193732. doi:10.1001/jamasurg.2019.3732
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3(2):114-122. doi:10.1016/S2213-8587(14)70229-3
- Su W, Huang J, Chen F, Mocarski M, Dall TM, Perrault L. Modeling the clinical and economic implications of obesity using microsimulation. J Med Econ. 2015;18(11):886-897. doi:10.3111/13696998.2015.1058805
- Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050-1061. doi:10.1002/oby.22794
- FDA approves new drug treatment for chronic weight management, first since 2014. Press release. FDA. June 4, 2021. Accessed July 22, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Davies M, Færch L, Jeppesen OK, et al; STEP 2 Study Group. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomized, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi:10.1016/S0140-6736(21)00213-0
- Wadden TA, Bailey TS, Billings LK, et al; STEP 3 Investigators. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi:10.1001/jama.2021.1831
- Rubino D, Abrahamsson N, Davies M, et al; Step 4 Investigators. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-1425. doi:10.1001/jama.2021.3224
- Kim N, Wang J, Burudpakdee C, et al. Cost-effectiveness analysis of semaglutide 2.4 mg for the treatment of adult patients with overweight and obesity in the United States. J Manag Care Spec Pharm. 2022;28(7):740-752. doi:10.18553/jmcp.2022.28.7.740

