- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Managed Care Considerations for the Treatment of Chronic Cough
ABSTRACT
Chronic cough (CC), defined as a daily cough lasting longer than 8 weeks in adults, is a common condition in the United States. CC is a diagnosis of exclusion associated with a substantial economic burden related to increased healthcare and medication utilization, decreased work productivity, a greater incidence of cough-related comorbidities, and reduced quality of life. CC treatment guidelines recommend stepwise treatment with specific nonpharmacologic therapies and pharmacologic agents. However, many patients may still have incomplete or no symptom relief, encounter response attenuation over time, or experience intolerable adverse effects. New targeted therapies for refractory CC are currently under development, including the purinergic 2X3 receptor antagonists gefapixant, BLU-5937, and sivopixant (S-600918) and the neurokinin-1 receptor antagonist orvepitant. These targeted agents may have improved efficacy and safety profiles, helping fill unmet treatment needs. If approved, managed care organizations must develop formulary placement and utilization management criteria based on clinical guideline recommendations, expert opinion, and cost-effectiveness analyses to support the clinically appropriate use of these targeted therapies for best patient outcomes.
Am J Manag Care. 2022;28(suppl 9):S166-S174. https://doi.org/10.37765/ajmc.2022.89245
Introduction
Chronic cough (CC) is a daily cough that persists longer than 8 weeks in adults and 4 weeks in children 14 years or younger.1-3 It is a commonly occurring condition, with an estimated prevalence of 10% to 15% in the United States, peaking in the fifth and sixth decade of life.2,4 CC affects women disproportionately, with about 67% of patients with CC being women. CC is a diagnosis of exclusion. Phenotypes of CC include asthmatic cough/eosinophilic bronchitis, reflux cough, postnasal drip/upper airway cough syndrome, and iatrogenic cough.2 In some cases, individuals experience persistent coughing, or refractory CC (RCC), regardless of the use of guideline-recommended treatment for presumed etiologies.5 Although unclear, it is believed CC may be caused by inflammation precipitated by low levels of innocuous stimuli, leading to increased sensitivity of the neuronal pathways involved in the cough reflex (cough hypersensitivity syndrome) and heightened laryngeal sensitivity.6 Delays in diagnosing RCC may occur due to recommended stepwise therapeutic algorithms, which exclude treatable causes first.7 According to the 2018 US National Medical Care Surveys, cough from any cause was the seventh principal reason for office visits and the fourth for emergency department visits.8,9
Economic Burden
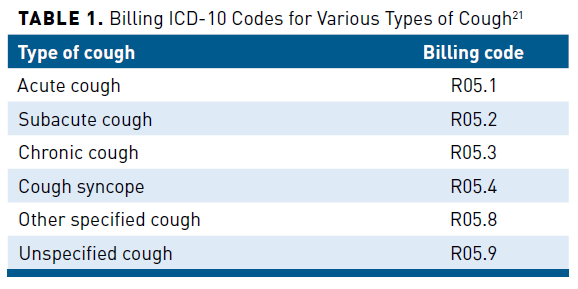
Studies have shown that CC is associated with substantial direct and indirect costs.10-19 In the United States, direct medical and pharmacy costs have been historically difficult to quantify based on administrative healthcare data due to the lack of specific International Classification of Diseases (ICD)-9 or ICD-10 diagnostic codes to distinguish patients with CC.20 Only as recently as October 1, 2021, have more specific billing ICD-10 codes become available to differentiate between types of cough, as shown in Table 1.21
Healthcare and Pharmacy Utilization
A cross-sectional, observational study compared medication and healthcare utilization of 10,926 participants aged 18 to 85 years at 1 year before their first outpatient visit to a CC specialist (index date) with a matched control group in the Kaiser Permanente Southern California (KPSC) Research Data Warehouse from 2013 to 2016. The patients were identified with a KPSC-specific CC encounter code. The study included participants who did not have an angiotensin-converting enzyme inhibitor dispensed in the year before the index date. The patients with CC had significantly (P <.001 for all) more medications for asthma, chronic obstructive pulmonary disease (COPD), rhinitis, gastroesophageal reflux disease (GERD), narcotic and non-narcotic antitussives, and other drugs to treat cough than the control group, except for theophylline (P = .074) and long-acting β-agonist monotherapy (P = .096) (see Figure 114). Healthcare utilization for respiratory-specific causes was significantly (all P <.001) greater for participants with CC than in the control group for outpatient visits (83.8% vs 26.8%), 1 or more emergency department visits (12.9% vs 5.0%), and 1 or more hospitalizations (5.6% vs 2.9%). Participants with CC consulted significantly (all P <.001) more specialists than the matched groups without CC (≥2 visits: 39.6% vs 5.7%; ≥3 visits: 11.0% vs 0.8%; ≥4 visits: 2.2% vs 0.1%). Laboratory testing was also significantly greater (all P <.001) in patients with CC than in participants in the control groups, including imaging such as chest x-ray, chest computed tomography (CT)/magnetic resonance imaging/ultrasound; gastrointestinal testing including esophageal pH studies/manometry, barium swallow or upper gastrointestinal, and esophageal endoscopy; respiratory tests including methacholine challenge, spirometry, bronchoscopy, laryngoscopy, sinus x-ray/CT, and nasal/sinus endoscopy; and allergy testing.14
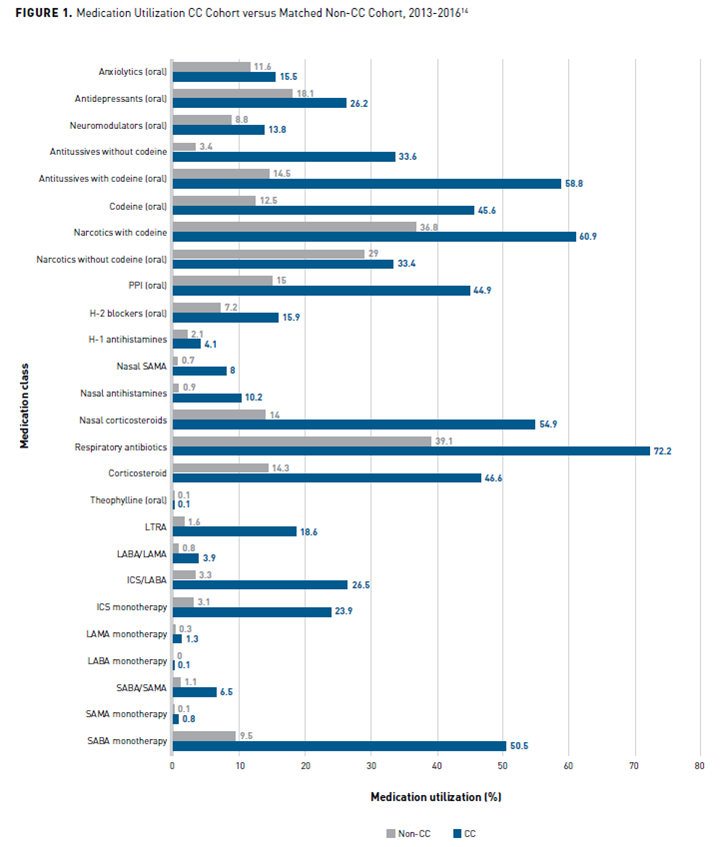
CC, chronic cough; GERD, gastroesophageal reflux disease; H1, histamine 1; H2, histamine 2; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; PPI, proton pump inhibitor; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist.
Direct Medical and Pharmacy Costs
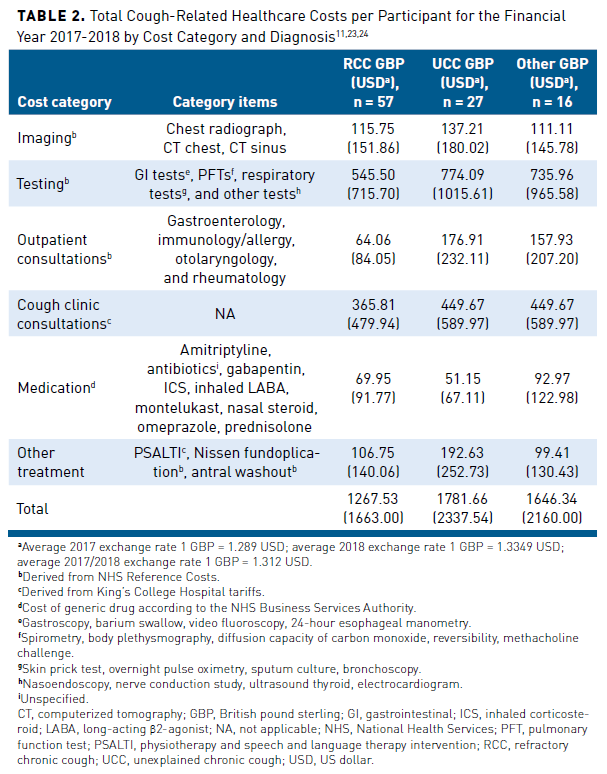
Studies conducted in other countries have assessed the economic burden of CC.10,11,18 Although the cost analyses of these studies may not directly translate to healthcare costs incurred for CC in the United States, they may provide some insight into the economic and clinical burden of patients with CC.22 A prospective study (N = 100) evaluated the healthcare utilization and cost in patients with CC seen at a specialist cough clinic at King’s College Hospital, London, United Kingdom, between 2016 and 2018. Researchers examined the effect of health status, objective cough frequency, cough severity, depression, and anxiety on measured costs. The study assessed health-related quality of life (HRQOL) using the 5-level EuroQol 5 dimension, cough-specific health status using the Leicester Cough Questionnaire (LCQ), 24-hour objective cough frequency using Leicester Cough Monitor, cough severity using visual analog scale, depression using Patient Health Questionnaire, and anxiety using Generalized Anxiety Disorder 7 (GAD7).The study estimated that the mean total cough-related healthcare cost for the 12 months before the cough clinic specialist visit was £645.73 GBP ($832.35 USD) per participant and £1017.12 GBP ($1357.75 USD) per participant for the 12 months after.23,24 Table 211,23,24 provides a breakdown of costs by category and diagnosis. Imaging, testing, and consultations accounted for most of the total cough-related healthcare costs; medication costs accounted for the least. Total costs for participants with unexplained CC (UCC) were significantly (P = .02) higher than those with RCC. After multivariate analysis, increased anxiety severity (GAD7) and poor cough-specific health status (LCQ) were associated with increased costs (P <.001 and P = .037, respectively).11
Indirect Costs and Patient Impact
Quality of Life/Absenteeism/Presenteeism
Some recent studies have evaluated the effect of CC on indirect costs such as HRQOL, absenteeism, presenteeism, and work productivity and activity impairment (WPAI).12,13,15,16 A study by the KPSC managed care organization specifically assessed the impact of CC on health status and QOL.13 Researchers analyzed survey responses (N = 565) of adult participants aged 18 to 85 years with CC on validated instruments that measured cough-related health status over the past 2 weeks (LCQ), symptom-specific health status via the cough hypersensitivity Hull Airway Reflux Questionnaire (HARQ), and QOL with Cough Quality of Life Questionnaire (CQLQ). Survey results showed participants with CC had greater cough hypersensitivity (mean HARQ total score: 33 ± 13.6; normal ≤13, a higher score is worse) and poorer QOL (mean CQLQ total score: 56.9 ± 17.5; range: 28 [no adverse effect (AE)] to 112 [worst impact]). Female respondents with CC experienced a more significant disease burden than men, with a greater average cough severity (5.4 vs 5.0; P = .046), worse LCQ scores (10.9 vs 12.5; P <.001), greater HARQ scores (34.5 vs 29.8; P <.001), and reduced CQLQ scores (58.8 vs 51.0; P <.001). Women also had an increased risk of certain comorbidities compared with men, such as asthma (relative risk ratio [RRR], 1.49; 95% CI, 1.15-1.92), allergic rhinitis (RRR, 1.75; 95% CI, 1.18-2.58), and any potential cough complications (RRR, 1.55; 95% CI, 1.01-2.39), but they experienced a decreased risk of COPD and lung diseases (RRR, 0.76; 95% CI, 0.61-0.94). However, men had a higher risk of mortality from comorbidities than women, reported on the Charlson Comorbidity Index at 2.0 versus 1.7, respectively (P = .02).13,25 Participants who were non-White reported a significantly (all P <.001) greater average cough severity than respondents who were White (5.7 vs 5.0), worse mean LCQ scores (10.3 vs 12.0), poorer HARQ (35.9 vs 31.6), and lower mean CQLQ total scores (61.3 vs 53.9). Analyses revealed strong correlations between LCQ and HARQ (−0.65), LCQ and CQLQ (−0.80), and HARQ and CQLQ (0.69).13
A Korean study analyzed data from 56,632 participants aged 40 years and older enrolled in the 2010 to 2016 Korean National Health and Nutrition Examination Survey and found that CC negatively impacted QOL. Participants included in the study responded to the 3-level EuroQoL 5-dimension (EQ-5D-3L) questionnaire and indicated the presence of CC. The EQ-5D-3L index score (0 = death, 1 = perfect health) measured HRQOL in 5 domains. Participants with CC had a significantly (P <.001) lower overall EQ-5D-3L index score than those without CC (0.79 vs 0.86). The average difference in overall EQ-5D-3L index score was the greatest in women aged 65 years and older, with an index score of 0.55 with CC versus 0.70 without CC (P <.001). Multivariate analyses adjusting for demographic factors and comorbidities including depression, arthritis, asthma, and COPD found that CC was significantly associated with QOL independent of these confounders. The presence of CC was also associated significantly with QOL across all 5 domains of anxiety/depression (odds ratio [OR], 1.77; 95% CI, 1.38-2.27; P <.001), usual activities (OR, 1.58; 95% CI, 1.21-2.07); P = .001), pain/discomfort (OR, 1.62; 95% CI, 1.32-2.00; P <.001), self-care (OR, 1.56; 95% CI, 1.09-2.23; P = .016), and mobility (OR, 1.31; 95% CI, 1.01-1.70; P = .042).12
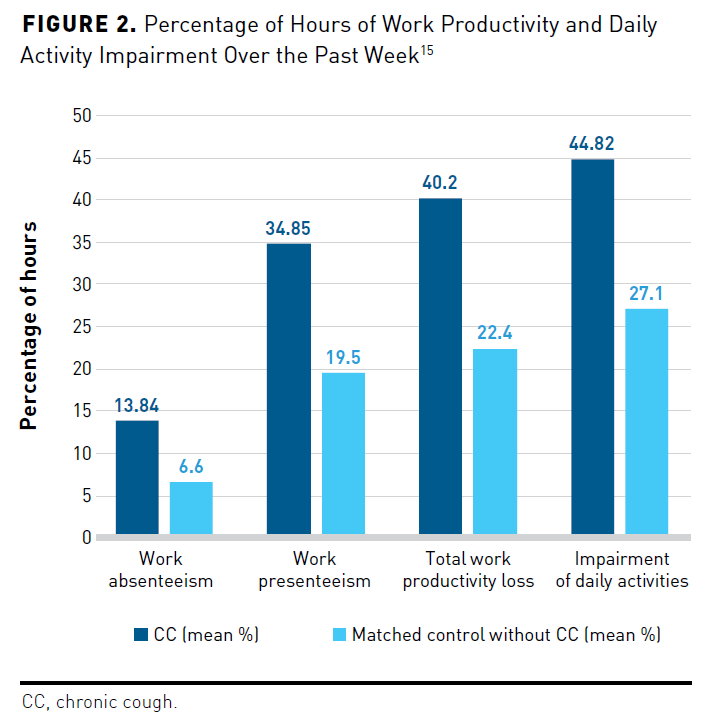
In addition to poorer QOL, a US study using National Health Wellness Survey data found that CC significantly impaired WPAI and increased rates of presenteeism and absenteeism.15 The researchers compared HRQOL scores on the Medical Outcomes Study 36-item Short Form Survey Version 2, on which a lower score reflects poorer health, and the EQ-5D-5L (0 = death, 1 = perfect health) of 3654 respondents aged 18 years and older with CC with 10,962 matched participants without CC. The group with CC had significantly (all P <.001) lower 36-Item Short Form Survey Version 2 mean scores across the 2 subcomponents of physical (42.87 vs 48.75) and mental (41.69 vs 47.37), Short Form 6-Item Descriptive Utility Index (0.62 vs 0.71; 0 = death, 1 = perfect health), and mean EQ-5D-5L scores (0.71 vs 0.81) compared with the group without CC. Participants with CC had greater anxiety in the prior 2 weeks compared with participants without CC, with mean GAD7 scores of 6.6 versus 3.8 (P <.001), respectively, in which scores between 15 and 21 indicate severe anxiety; they also reported greater depression on the Patient Health Questionnaire-9 score (8.8 vs 5.1; P <.001), in which scores from 20 to 27 reflect severe depression. Compared with matched controls, respondents with CC had more than double the incidence of severe anxiety (13.1% vs 5.5%; P <.001) and severe depression (9.8% vs 4.1%; P <.001). The CC cohort experienced significantly (all P <.001) more impairment of daily activities (44.8% vs 27.1%), absenteeism (13.8% vs 6.6%), presenteeism (34.9% vs 19.5%), and total work productivity loss (40.2% vs 22.4%) than the control cohort (Figure 215).15
A smaller cross-sectional study in Japan also revealed that CC negatively impacted HRQOL, WPAI, absenteeism, and presenteeism. The 568 respondents with CC reported significantly poorer HRQOL compared with 2272 matched participants without CC in the 12-Item Short Form Survey Instrument Version 2 and subcomponents (mental component summary mean scores: 46.27 vs 49.22; physical component summary mean scores: 49.10 vs 52.03; both P <.001) as well as in the Short Form 6-Item Descriptive Utility Index (0.72 vs 0.77; mean EQ-5D-5L: 0.80 vs 0.87; both P <.001) after propensity score matching. Compared with the control group, respondents with CC reported more symptoms of depression (8.3% vs 4.1%; P <.001), anxiety (3.9% vs 1.5%; P <.001), and sleep disturbances (66.2% vs 48.7%; P <.001). Participants with CC also reported significantly greater total activity impairment (28.05% vs 19.01%; P <.001), total work productivity impairment (26.48% vs 18.49%, P <.001), absenteeism (4.54% vs 2.42%; P = .004), and presenteeism (25.56% vs 17.38%; P <.001) than the matched control cohort.16
Family Quality of Life
Although previous studies have shown that CC significantly affects patient QOL, fewer studies have examined the effect on family QOL.17,19 A 2008 study of parents of children recently referred for evaluation to the Royal Children’s Hospital, Melbourne, Australia, found that CC in children is also burdensome to parents. In the previous 12 months, parents consulted multiple medical practitioners for their child’s CC with 76% consulting up to 3 providers and 30.5% consulting 4 or more providers; 80% of the children had 5 or more doctor visits for cough and 53% had 10 or more visits. The number of doctor visits in the past 12 months; the 21-item self-reported Depression, Anxiety, and Stress Scale total scores (higher scores equal greater severity); and subjective cough scores, which ranged from 0, or no cough, to 5, or cannot perform most activities, negatively correlated to the parent cough-specific QOL clinical impact and psychometric total burden scores. Parent QOL was significantly lower in the group whose children were still coughing (n = 32) compared with those who had stopped coughing at follow-up (n = 49; parent cough-specific QOL clinical impact: 4.78 vs 6.93; parent cough-specific QOL psychometric total burden score: 5.48 vs 6.88; both P = .001).17
A multicenter, randomized controlled study in Australia evaluated the effect of a standardized treatment algorithm or usual care for the treatment of 272 children with CC on parent cough-specific (PC-QOL) and patient QOL (PedsQL). A significantly greater proportion of children were cough free at week 6 in the early standardized treatment arm than in the delayed usual care arm (differences in proportions, 0.247; 95% CI, 0.134-0.361; P = .0001). Treatment significantly improved parent QOL at 6 weeks compared with baseline for the entire cohort (median PC-QOL scores 3.6 vs 6.5; P = .001; median PedsQL score 78.3 vs 92.5; P = .0001). At 6 weeks, the mean PC-QOL difference between the early arm and delayed arm cohort was 0.6 (95% CI, 0.29-1.0), which indicated a small but statistically insignificant improvement in parental QOL.26
Additional Comorbidities and Complications
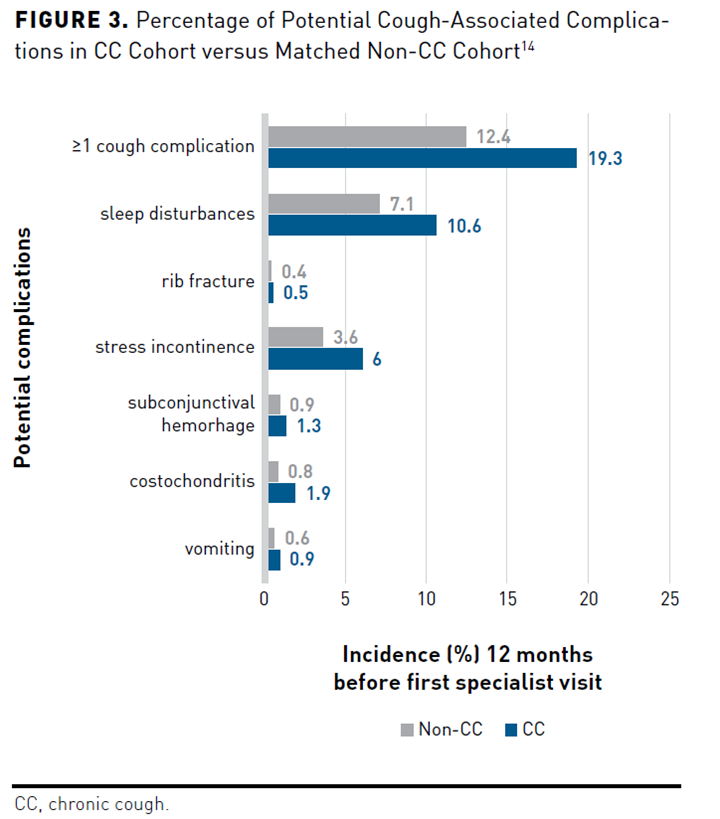
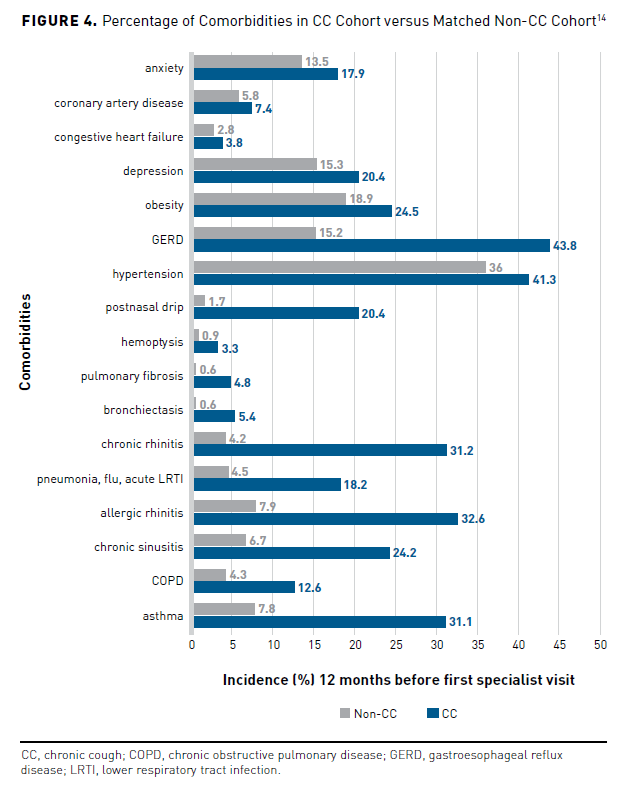
In addition to the negative effects on WPAI, presenteeism, absenteeism, and patient and family QOL, people with CC also have an increased burden of CC-related complications and comorbidities. A KPSC cross-sectional, observational study compared the incidence of comorbidities and complications of participants with CC (n = 10,926) with a matched control group (n = 10,926) 12 months before an initial specialist visit. Potential cough-associated complications occurred in 19.3% of participants in the CC group versus the non-CC group (12.4%; P <.001). Patients with CC experienced more sleep disturbances (P <.001), stress incontinence (P <.001), costochondritis (P <.001), subconjunctival hemorrhage (P = .007), vomiting (P = .002), rib fracture (P = .148), and 1 or more potential cough complications (P <.001) than did patients without CC (Figure 314). Respiratory comorbidities occurring more often in the CC group vs the non-CC group (all P <.001) included asthma, COPD, chronic sinusitis, allergic rhinitis, pneumonia, influenza, acute lower respiratory tract infections, chronic rhinitis, bronchiectasis, pulmonary fibrosis, hemoptysis, and postnasal drip (Figure 414). Patients in the CC group experienced nonrespiratory disorders more frequently than those without CC (all P <.001), including hypertension, GERD, obesity, depression, congestive heart failure, coronary artery disease, and anxiety.14
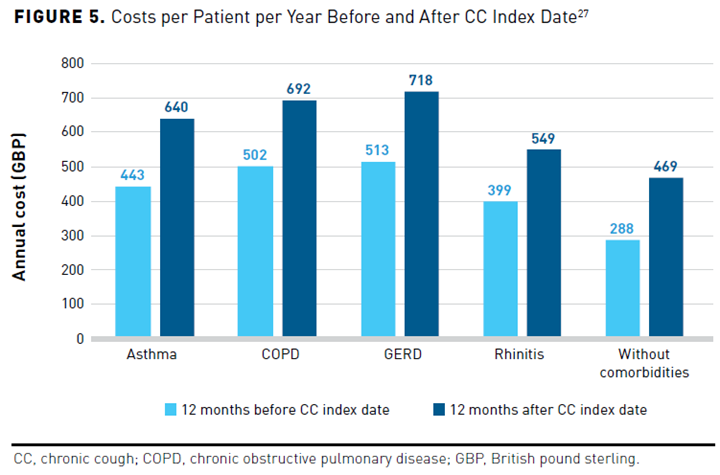
A cross-sectional, retrospective study in the United Kingdom examined the annual estimated outpatient treatment costs related to CC alone versus CC with comorbid conditions. The study identified 43,453 participants aged 18 years and older with CC using ICD-10 codes and prescription claims data from the Discover dataset between January 1, 2015, and September 30, 2019. The study determined costs related to asthma, COPD, GERD, and rhinitis for 12 months before and after the index date, which was defined as 2 or more documented cough-related consultations for symptoms persisting at least 8 weeks. Overall, 68% of study participants had at least 1 CC-related comorbidity. Annual costs per patient were higher in patients with CC and a comorbidity than in those without (Figure 527). Patients with CC and GERD incurred the greatest costs, followed by CC and COPD, then CC and asthma, and lastly CC and rhinitis. The total annual outpatient costs per patient increased 31% for those with CC and asthma (n = 8834), 27% for those with CC and COPD (n = 6105), 29% for those with CC and GERD (n = 5217), and 27% for those with CC and rhinitis (n = 3685) from 12 months before and after the index date.27
Targeted Therapy Managed Care Considerations
The Current Standard of Care
Guidelines recommend stepwise treatment with specific nonpharmacologic therapies such as multimodality speech, pathology therapy, and pharmacologic agents, depending on the suspected trigger.1-3,28 The American College of Chest Physicians guidelines do not recommend the use of over-the-counter medications to treat CC in children due to their lack of efficacy and AE potential.1 Potential guideline-recommended medication options include antibiotics; neuromodulators such as amitriptyline, baclofen, gabapentin, pregabalin, and low-dose morphine; nalbuphine; proton pump inhibitors (PPIs); and inhaled corticosteroids (ICS).1-3,28 Although there are various therapy options, it is estimated that 12% to 50% of patients with CC develop RCC.29 Studies have demonstrated this unmet treatment need that is further complicated by heterogeneous CC phenotypes in patients.2,7,13,29-32 Treatments such as antibiotics, PPIs, and ICS will only be efficacious for a specific phenotype (eg, PPI for CC and GERD).5 Most of the current pharmacologic treatment options are off label, with low to moderate levels of evidence.2,7 An internet survey of 1120 participants with CC from 29 European countries reported that medications had limited effect in 57% of respondents or no response in 36%.30 Some small studies have shown that people with UCC who are treated with neuromodulators, such as amitriptyline, desipramine, gabapentin, nortriptyline, and tramadol, may experience tachyphylaxis in response to long-term treatment.29,32 Neuromodulator use may be associated with intolerable adverse reactions, such as fatigue, dizziness, xerostomia, and sedation, leading to treatment discontinuation.5,7 Additionally, in some US states, morphine, pregabalin, and gabapentin are controlled substances as they have the potential for increased risk of abuse or overdose, which may limit patient access.33-35
Efficacy of Targeted Therapy in Resolving or Mitigating Chronic Cough
New targeted therapies currently under development for treating RCC include the purinergic 2X3 (P2X3) receptor antagonists gefapixant, BLU-5937, and sivopixant (S-600918) and the neurokinin-1 (NK-1) receptor antagonist orvepitant.5,7 These agents have shown some efficacy in the reduction in objective daytime cough frequency, improvement in visual analog scale, and patient-reported LCQ in phase 2 and 3 clinical trials in participants with RCC and/or UCC.36-40 Of these agents, gefapixant is furthest along in clinical development; however, the FDA declined approval of a New Drug Application for gefapixant in early 2022, requesting additional information related to efficacy measurement.41 Additional phase 2 trial development of BAY 1817080 (eliapixant), another P2X3 receptor antagonist that had demonstrated some initial promise for the treatment of RCC in a phase 2a trial, was halted by the manufacturer due to unfavorable overall benefit-versus-risk profile.42,43 Additional data will be needed to evaluate future support for this class of drugs.
Adverse Effects of Targeted Therapy
Phase 2 and 3 data with targeted therapies did not reveal significant safety concerns.5,7 Some AEs that occurred more often with the NK-1 receptor antagonist orvepitant than placebo in the unpublished VOLANO-2 study were back pain, headache, dizziness, and oropharyngeal pain.44 The P2X3 antagonist trials reported AEs involving taste alterations.5,7 The majority were mild, and most participants did not discontinue treatment due to these taste disturbances. These are thought to be mechanism-related, with the agents having greater selectivity reporting less of an effect. Taste disturbance AEs were reported more often with gefapixant in COUGH-1 and COUGH-2, including ageusia (4.9% vs 6.5%), dysgeusia (16.2% vs 21.1%), hypergeusia (0.4% vs 0.5%), hypogeusia (2.6% vs 6.1%), and taste disorder (3.8 vs 3.5%), respectively.40 In the RELIEF study, dysgeusia (8.2%) and hypogeusia (3.3%) occurred in participants receiving BLU-5937, while dysgeusia and hypogeusia both occurred in 3.2% of participants receiving sivopixant.38,39
Strategies for Targeted Therapies
Currently, the medications used to treat CC are primarily unmanaged prescription drugs or over-the-counter medications, and direct medication costs are a minor contributor to the overall direct costs of CC treatment.11 If these targeted therapies are approved, new utilization management criteria will be initially based on expert opinion, as they will be first-in-class agents; clinical guidelines currently do not include recommendations concerning these agents. The Pharmacy and Therapeutics Committee will need to evaluate the cost, efficacy, and safety of targeted therapy compared with current guideline-recommended therapy to determine formulary placement and appropriate utilization management.45 Current treatment for CC is only partially effective or ineffective for many patients and may have undesirable AEs.5,7
Cost-effectiveness analyses should assess therapy value, accounting for cost savings in direct healthcare utilization, reduced costs of CC-related complications and comorbidities, decreased costs incurred from AEs or ineffective medication options, improved work productivity, and better QOL in addition to the comparative medication costs.45 Data from phase 2 and 3 trials with targeted therapies have demonstrated some efficacy and safety advantages for the treatment of RCC and UCC.37-40 However, there is a lack of head-to-head randomized controlled studies of targeted therapy with standard of care to determine the comparative safety and efficacy. Long-term safety and efficacy data with targeted therapies are not yet available.
Managed care organizations may use prior authorization to manage this class appropriately.46 Potential drug prior authorization criteria may require RCC diagnosis by a cough clinic specialist with documentation of clinical control of underlying conditions and adequate diagnostic workup eliminating other potentially treatable causes of cough (eg, stable pulmonary function tests, normal chest x-ray/CT scan reading).1-3,28 Other prior authorization requirements may include documentation of nonsmoking status for a clinically appropriate amount of time and the absence of medications that may cause a drug-induced cough (eg, angiotensin-converting enzyme inhibitors, sitagliptin). Trial of treatment of generic neuromodulators (eg, gabapentin, amitriptyline) and speech and language therapy recommended by the current clinical guidelines, in the absence of contraindications, may be appropriate requirements before coverage of targeted therapies.
A comprehensive medication review of drug therapy through a medication therapy management program or other drug utilization review may be helpful to potentially identify other treatable disease states exacerbating or causing cough or to eliminate iatrogenic causes of cough.1-3,28,47,48 The incidence of taste alterations with P2X3 antagonists may lead to premature treatment discontinuation, so it is imperative that patients be counseled about these potential AEs.5,7 Managed care organizations may implement clinical programs to encourage the proper use of targeted therapies to help maximize their benefits and optimize outcomes.49
Conclusions
CC is a condition associated with increased healthcare costs and can impact patient and family QOL. Current guideline-recommended therapy is often ineffective for many patients with RCC. New targeted therapies, such as P2X3-receptor antagonists and an NK-1 receptor antagonist, are under development and may have improved efficacy and tolerability for the treatment of RCC. However, there is a lack of active comparator studies with these agents as well as an absence of long-term safety and efficacy data. Managed care organizations must develop sound utilization management criteria based on clinical guideline recommendations, expert opinion, and cost-effectiveness analyses to support appropriate use and optimize patient outcomes. If these agents are approved, they may help fill the significant unmet treatment needs of patients with RCC.
Author affiliation: Douglas S. Burgoyne, PharmD, FAMCP, is an Adjunct Associate Professor, Department of Pharmacotherapy, at the University of Utah College of Pharmacy, Salt Lake City, UT.
Funding source: This activity is supported by an educational grant from Merck Sharp & Dohme Corp.
Author disclosure: Dr Burgoyne has no relevant financial relationships with commercial interests to disclose.
Authorship information: Substantial contributions to acquisition of data; analysis and interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content.
Address correspondence to: doug.burgoyne@utah.edu
Medical writing and editorial support provided by: Lori Uildriks, PharmD
REFERENCES
- Chang AB, Oppenheimer JJ, Irwin RS; CHEST Expert Cough Panel. Managing chronic cough as a symptom in children and management algorithms: CHEST Guideline and Expert Panel Report. Chest. 2020;158(1):303-329. doi:10.1016/j.chest.2020.01.042
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136. doi:10.1183/13993003.01136-2019
- Irwin RS, French CL, Chang AB, Altman KW; CHEST Expert Cough Panel. Classification of cough as a symptom in adults and management algorithms: CHEST Guideline and Expert Panel Report. Chest. 2018;153(1):196-209. doi:10.1016/j.chest.2017.10.016
- Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479-1481. doi:10.1183/09031936.00218714
- Mazzone SB, McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin Pharmacol Ther. 2021;109(3):619-636. doi:10.1002/cpt.2003
- McGarvey L, Gibson PG. What is chronic cough? terminology. J Allergy Clin Immunol Pract. 2019;7(6):1711-1714. doi:10.1016/j.jaip.2019.04.012
- Morice A, Dicpinigaitis P, McGarvey L, Birring SS. Chronic cough: new insights and future prospects. Eur Respir Rev. 2021;30(162):210127. doi:10.1183/16000617.0127-2021
- Santo L, Okeyode T. National Ambulatory Medical Care Survey: 2018 National Summary Tables. Centers for Disease Control and Prevention. Accessed July 13, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2018-namcs-web-tables-508.pdf
- Cairns C, Kang K, Santo L. National Hospital Ambulatory Medical Care Survey: 2018 Emergency Department Summary Tables. Centers for Disease Control and Prevention. Accessed July 13, 2022.
www.cdc.gov/nchs/data/nhamcs/web_tables/2018-ed-web-tables-508.pdf - Sichali JM, Khan JAK, Gama EM, et al. Direct costs of illness of patients with chronic cough in rural Malawi-experiences from Dowa and Ntchisi districts. PLoS One. 2019;14(12):e0225712. doi:10.1371/journal.pone.0225712
- Cho PS, Shearer J, Simpson A, Campbell S, Pennington M, Birring SS. Healthcare utilization and costs in chronic cough. Curr Med Res Opin. 2022;38(7):1-35. doi:10.1080/03007995.2022.2065142
- Won HK, Lee JH, An J, et al. Impact of chronic cough on health-related quality of life in the Korean adult general population: the Korean National Health and Nutrition Examination Survey 2010-2016. Allergy Asthma Immunol Res. 2020;12(6):964-979. doi:10.4168/aair.2020.12.6.964
- Zeiger RS, Schatz M, Hong B, et al. Patient-reported burden of chronic cough in a managed care organization. J Allergy Clin Immunol Pract. 2021;9(4):1624-1637.e10. doi:10.1016/j.jaip.2020.11.018
- Zeiger RS, Schatz M, Butler RK, Weaver JP, Chen W. Characteristics and burden of chronic cough in adults in a large managed care organization. Presented at the American Thoracic Society Conference; May 17-22, 2019. www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4622
- Meltzer EO, Zeiger RS, Dicpinigaitis P, et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract. 2021;9(11):4037-4044.e2. doi:10.1016/j.jaip.2021.07.022
- Kubo T, Tobe K, Okuyama K, et al. Disease burden and quality of life of patients with chronic cough in Japan: a population-based cross-sectional survey. BMJ Open Respir Res. 2021;8(1):e000764. doi:10.1136/bmjresp-2020-000764
- Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. What is the burden of chronic cough for families? Chest. 2008;134(2):303-309. doi:10.1378/chest.07-2236
- Birring SS, Currie CJ, Holden SE, et al. The financial burden of treating patients presenting with acute and chronic cough. Curr Med Res Opin. 2021;37(12):2175-2184. doi:10.1080/03007995.2021.1982685
- Waring G, Kirk S, Fallon D. The impact of chronic non-specific cough on children and their families: a narrative literature review. J Child Health Care. 2020;24(1):143-160. doi:10.1177/1367493518814925
- Zeiger RS, Xie F, Schatz M, et al. Prevalence and characteristics of chronic cough in adults identified by administrative data. Perm J. 2020;24:1-3. doi:10.7812/TPP/20.022
- Moore K. New diagnosis codes effective Oct. 1. here are some family physicians should know. AAFP. Published August 1, 2021. Accessed July 13, 2022. www.aafp.org/journals/fpm/blogs/gettingpaid/entry/new_diagnosis_codes.html
- Kamal R, Cox C. How do healthcare prices and use in the U.S. compare to other countries? Peterson-KFF Health System Tracker. Published May 2, 2018. Accessed July 13, 2022. www.healthsystemtracker.org/chart-collection/how-do-healthcare-prices-and-use-in-the-u-s-compare-to-other-countries/
- British pound to US dollar spot exchange rates for 2017. Exchange Rates UK. Accessed July 13, 2022. www.exchangerates.org.uk/GBP-USD-spot-exchange-rates-history-2017.html
- British pound to US dollar spot exchange rates for 2018. Exchange Rates UK. Accessed July 13, 2022. www.exchangerates.org.uk/GBP-USD-spot-exchange-rates-history-2018.html
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. doi:10.1016/0021-9681(87)90171-8
- Chang AB, Robertson CF, van Asperen PP, et al. A cough algorithm for chronic cough in children: a multicenter, randomized controlled study. Pediatrics. 2013;131(5):e1576-1583. doi:10.1542/peds.2012-3318
- Hull JH, Langerman H, Ul-Haq Z, Kamalati T, Lucas A, Levy M. Burden and impact of chronic cough in UK primary care: a dataset analysis. BMJ Open. 2021;11(12):e054832. doi:10.1136/ bmjopen-2021-054832
- Gibson P, Wang G, McGarvey L, Vertigan AE, Altman KW, Birring SS; CHEST Expert Cough Panel. Treatment of unexplained chronic cough: CHEST Guideline and Expert Panel Report. Chest. 2016;149(1):27-44. doi:10.1378/chest.15-1496
- Bowen AJ, Nowacki AS, Contrera K, et al. Short- and long-term effects of neuromodulators for unexplained chronic cough. Otolaryngol Head Neck Surg. 2018;159(3):508-515. doi:10.1177/0194599818768517
- Chamberlain SA, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193(3):401-418. doi:10.1007/s00408-015-9701-2
- Kang SY, Won HK, Lee SM, et al. Impact of cough and unmet needs in chronic cough: a survey of patients in Korea. Lung. 2019;197(5):635-639. doi:10.1007/s00408-019-00258-9
- Bowen AJ, Huang TL, Nowacki AS, et al. Tachyphylaxis and dependence in pharmacotherapy for unexplained chronic cough. Otolaryngol Head Neck Surg. 2018;159(4):705-711. doi:10.1177/0194599818788062
- Lyrica. Prescribing information. Pfizer; 2020. Accessed July 13, 2022. labeling.pfizer.com/showlabeling.aspx?id=561
- Collins S. More states make gabapentin a Schedule V controlled substance. Pharmacy Today. 2021;27(10):33. doi:10.1016/j.ptdy.2021.09.016
- Controlled substance schedules. US Department of Justice Drug Enforcement Administration Diversion Control Division. Accessed July 13, 2022. www.deadiversion.usdoj.gov/schedules/
- Smith J, Allman D, Badri H, et al. The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1). Chest. 2020;157(1):111-118. doi:10.1016/j.chest.2019.08.001
- NeRRE Therapeutics announces positive findings from phase 2b study with orvepitant in chronic cough. News release. GlobeNewswire; June 7, 2019. Accessed July 13, 2022. www.globenewswire.com/news-release/2019/06/07/1865819/0/en/NeRRe-Therapeutics-announces-positive-findings-from-Phase-2b-study-with-orvepitant-in-chronic-cough.html
- Niimi A, Saito J, Kamei T, et al. Randomised trial of the P2X3 receptor antagonist sivopixant for refractory chronic cough. Eur Respir J. 2022;59(6):2100725. doi:10.1183/13993003.00725-2021
- Bonuccelli CM. Update on the development of BLU-5937 for the treatment of refractory chronic cough. Bellus Health; June 12, 2021. Accessed July 13, 2022. bellushealth.com/wp-content/uploads/Bellus-Health-ACC2021-Update-on-BLU-5937-in-RCC.pdf
- McGarvey LP, Birring SS, Morice AH, et al; COUGH-1 and COUGH-2 Investigators. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022;399(10328):909-923. doi:10.1016/S0140-6736(21)02348-5
- Merck provides U.S. and Japan regulatory update for gefapixant. News release; Merck. January 24, 2022. Accessed August 22, 2022. www.merck.com/news/merck-provides-u-s-and-japan-regulatory-update-for-gefapixant/
- Bayer will discontinue phase II development candidate eliapixant. News release. Bayer; February 4, 2022. Accessed July 13, 2022. media.bayer.com/baynews/baynews.nsf/id/8D5D4D684755D4C5C12587DF003C4A54?open&ref=irrefndcd
- Morice A, Smith JA, McGarvey L, et al. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J. 2021;58(5):2004240. doi:10.1183/13993003.04240-2020
- A dose-ranging study of orvepitant in patients with chronic refractory cough. Clinical Trials.gov identifier: NCT02993822. Updated April 26, 2022. Accessed July 13, 2022. clinicaltrials.gov/ct2/show/results/NCT02993822
- AMCP Partnership Forum: principles for sound Pharmacy and Therapeutics (P&T) Committee practices: what’s next? J Manag Care Spec Pharm. 2020;26(1):48-53. doi:10.18553/jmcp.2020.26.1.48
- Prior authorization. Academy of Managed Care Pharmacy. Published July 18, 2019. Accessed July 13, 2022. www.amcp.org/about/managed-care-pharmacy-101/concepts-managed-care-pharmacy/prior-authorization
- Ferries E, Dye JT, Hall B, Ndehi L, Schwab P, Vaccaro J. Comparison of medication therapy management services and their effects on health care utilization and medication adherence. J Manag Care Spec Pharm. 2019;25(6):688-695. doi:10.18553/jmcp.2019.25.6.688
- Drug utilization review. Academy of Managed Care Pharmacy. Published July 18, 2019. Accessed July 13, 2022. www.amcp.org/about/managed-care-pharmacy-101/concepts-managed-care-pharmacy/drug-utilization-review
- Managed care pharmacy tools. Academy of Managed Care Pharmacy. Published August 12, 2019. Accessed July 13, 2022. www.amcp.org/policy-advocacy/policy-advocacy-focus-areas/amcp-policy-digest/managed-care-pharmacy-tools
