- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Key Considerations for Optimizing B-VEC in Dystrophic Epidermolysis Bullosa
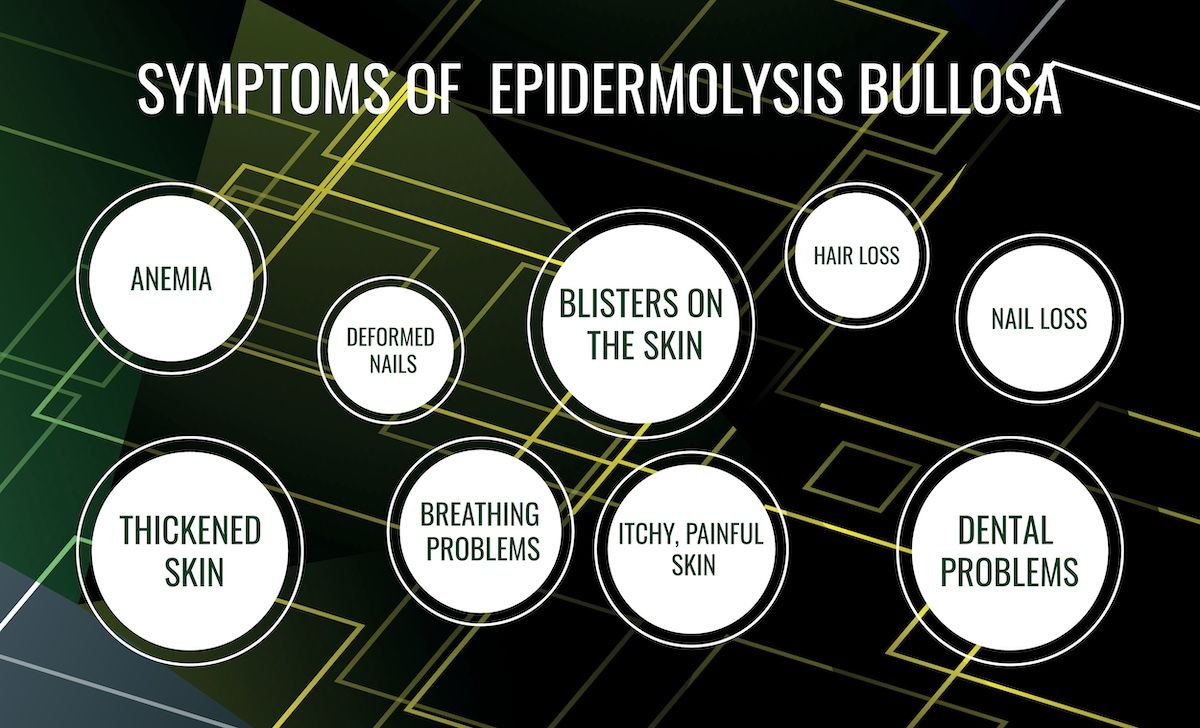
Beremagene geperpavec-svdt (B-VEC) entered the market in 2023 as the first approved corrective treatment for dystrophic epidermolysis bullosa (DEB), a rare genetic disease affecting the skin and nails that is caused by mutations in the COL7A1 gene.
Following the recent approval of the first gene therapy for dystrophic epidermolysis bullosa (DEB), researchers of a new study in Journal of Dermatological Treatment have outlined important considerations of administration, aiming to help optimize treatment and patient outcomes.1
Beremagene geperpavec-svdt (B-VEC) entered the market in 2023 as the first approved corrective treatment for DEB, a rare genetic disease affecting the skin and nails that is caused by mutations in the COL7A1 gene.2 In a phase 3 trial of B-VEC, a herpes-simplex virus type 1 vector-based gene therapy, the treatment was associated with greater wound healing at 3 and 6 months compared with placebo. Adverse effects included pruritus and chills.3
“The burden of wounds for the DEB community is immense. Painful blistering and open wounds interfere with the ability of DEB patients to perform daily activities, such as bathing, sleeping, and physical exercise,” wrote the researchers. “Patients and caregivers spend hours every day changing wound dressings, a process that is costly and uncomfortable. Concerns over life-threatening infections and development of highly aggressive [squamous cell carcinomas] are at the forefront of DEB management, with a focus on monitoring and prevention. B-VEC fundamentally addresses the defect underlying the wound burden of DEB patients by delivering full-length human COL7 protein to wounds and promoting their durable closure.”
Dystrophic epidermolysis bullosa is a rare genetic disease caused by mutations in the COL7A1 gene | Image Credit: ангелина ковальчук-stock.adobe.com
The novel treatment, which encodes 2 copies of full-length human COL7A1to restore COL7 protein, allows for flexible administration, as it is able to be given in a clinical setting or at home. As uptake of this novel treatment continues, researchers outlined various considerations for each step of treatment.
Prior to treatment, experts encourage determining which wounds to treat, proper cleaning of the areas, looking for infection, and making sure the patient is comfortable. When assessing which wounds to treat, those that pose the greatest impact on the patient’s daily life are often prioritized, as the initial weekly dose may not be able to address all wounds. Age was also highlighted by the researchers. For example, with infants, providers may prioritize treating wounds on hands or feet to mitigate risk of future deformity or loss of function.
Saline wipes and rinses were used in the phase 3 trial to cleanse the skin of patients prior to treatment, and various gentle cleaners and saline-based options can be used for bathing, such as diluted bleach in bathwater. Redness, pain, odor, and exudate are all signs of infection in wounds.
If the temperature is uncomfortable, B-VEC can be left at room temperature for 3 to 5 minutes before administration, and the tip of the syringe should not come in contact with the skin.
“The HCP [health care practitioner] may be tempted to reduce the interval between wound treatments to once every 2 or 3 weeks compared to the standard once per week. However, B-VEC administration is optimized for once-a-week use,” explained the researchers. “Longer intervals between B-VEC treatments may result in portions of healed wounds not receiving molecular correction. Thus, the HCP should attempt to follow weekly dosing when wounds are present. There is no benefit in applying B-VEC once wounds are closed; however, if a previously treated wound reopens, it should be prioritized for treatment until it closes again.”
Following treatment, a hydrophobic layer should be applied to the wound, either using a soft plastic or silicone-based covering, as the first layer and should remain on for about 1 day after administration.
References
1. Paller AS, Guide SV, Ayala D, et al. Practical considerations relevant to treatment with the gene therapy beremagene geperpavec-svdt for dystrophic epidermolysis bullosa. J Dermatol Treat. Published online May 9, 2024. doi:10.1080/09546634.2024.2350232
2. FDA approves first topical gene therapy for treatment of wounds in patients with dystrophic epidermolysis bullosa. News release. FDA. Published May 19, 2023. Accessed June 10, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-topical-gene-therapy-treatment-wounds-patients-dystrophic-epidermolysis-bullosa
3. Guide SV, Gonzalez ME, Bagci IS, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. New Engl J Med. 2022;387(24):2211-2219. doi:10.1056/NEJMoa2206663
