- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Increasing Clinical Awareness of Obesity as a Serious, Chronic, Relapsing, and Treatable Disease
ABSTRACT
The American Medical Association recognized obesity as a disease in 2013. Obesity is influenced by genetic, environmental, physiologic, behavioral, and sleep factors and is associated with approximately 200 health conditions. Assessed using body mass index, body composition, and evaluation of weight-related complications, obesity is treated with lifestyle interventions, anti-obesity medications, and metabolic and bariatric surgery. The age-adjusted prevalence of overweight and obesity in US adults has increased substantially in the 21st century, from an estimated 56% in 1988-1994 to approximately 73.1% in 2017-2018. Nevertheless, there are substantial barriers to successful obesity treatment in the United States, including inadequate treatment coverage; a lack of acceptance by providers, patients, and employers that obesity is a disease; the perception that treatment is ineffective; and the belief that obesity is a behavioral concern related to a lack of willpower. Obesity is a serious, chronic, relapsing, and treatable disease associated with many related conditions; it requires long-term medical management and multimodal care strategies.
Am J Manag Care. 2022;28(suppl 15):S271-S278. doi:10.37765/ajmc.2022.89290
For author information and disclosures, see end of text.
Introduction
Understanding of obesity has changed dramatically since the American Medical Association (AMA) recognized it as a disease in 2013.1 The many associations that have been identified between obesity-related genes and biologic processes highlight its physiologic basis.2-4 Intrinsic factors that lead to obesity are now meeting with an increasingly obesogenic environment, and obesity can be considered to be a global pandemic.5-7 The latest data from the World Health Organization demonstrate that globally, 13% of adults had obesity in 2016.8 This prevalence has almost tripled since 1975.7,8 In the United States, approximately 100 million people had obesity in 2016.9
As the burdens of this disease grow, so does the need for up-to-date review of clinical guidelines and scholarly research. This first article of the supplement reinforces current medical understanding of obesity as a serious, chronic, relapsing, and treatable disease. In the supplement’s second article, Danielle C. Massie, PharmD, and others describe obesity’s clinical and economic burden.10 Marc-André Cornier, MD, summarizes current guidelines for the treatment of adults with obesity in the supplement’s third article, and, in the fourth article, Janine Kyrillos, MD, presents a novel anti-obesity medication (AOM) option in semaglutide 2.4-mg injection (Wegovy®; Novo Nordisk).11,12 Finally, in the fifth article of this supplement, Anastassia Amaro, MD, and colleagues discuss managed care considerations for obesity.13 Obesity affects people of all ages. This supplement addresses the management of obesity in adults.
Background on Obesity
Obesity meets AMA criteria for a disease: causing impairment of the normal functioning of part or parts of the body, possessing characteristic signs or symptoms, and leading to harm or morbidity.1 The disease is associated with dysfunction of the tightly regulated energy homeostasis system, in that an accumulation of excess adipose tissue impairs normal hormonal and metabolic function through endocrine dysfunction; this causes dysregulated adipokine signaling, abnormal endothelial function, infertility, blood pressure elevation, nonalcoholic fatty liver disease, dyslipidemia, and systemic and adipose tissue inflammation.1,14-16 The development of obesity is influenced by a variety of factors, including physiologic factors, genetics, associated health conditions, behavioral factors, race/ethnicity, gender, age, childbearing status, adverse childhood experiences, and social determinants of health including socioeconomic status and the built environment.1,15,17-20
Physiologic factors
Body weight is normally regulated through a complex relationship of genetics, environment, appetite, metabolism, hormones, and energy expenditure.14,21,22 One of the main regulators of appetite is the hypothalamus, which coordinates signals from the brain, the peripheral circulation, and the gastrointestinal (GI) tract to regulate energy intake and expenditure.14 Peptide neurotransmitters in the hypothalamus’s arcuate nucleus are associated with appetite; these peptides (ie, neuropeptide, agouti-related peptide, proopiomelanocortin, cocaine- and amphetamine-regulated transcript) induce or inhibit eating.14 Neurons that express these peptides communicate with signals from all over the body, including mechano- and chemoreceptors, nutrients (eg, glucose, amino acids, and fatty acids), GI peptide hormones (ie, cholecystokinin, ghrelin), hormones (eg, insulin, leptin, and adiponectin), and each other to influence hunger, feeding, and energy expenditure.14
GI hormones also are involved in appetite regulation. Gut hormones act on digestion and absorption of nutrients through GI motility and secretion.14 Gut peptides (eg, cholecystokinin, pancreatic polypeptide, peptide YY [PYY], glucagon-like peptide-1 [GLP-1], glucose-dependent insulinotropic polypeptide, oxyntomodulin, ghrelin) influence energy intake.14,23 Metabolic adaptations that occur after weight loss may result in increased signals for energy intake due to changes in appetite-regulating hormones.21,24 Metabolic adaptations can affect long-term weight loss maintenance; the “set-point” theory of body weight posits that the body has an internal physiologic mechanism to regulate metabolism and maintain body weight within a predetermined, stable range.14,24 Such changes may explain why many metabolic and bariatric surgeries, such as sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB), often are so successful at supporting weight loss—such procedures not only mechanically restrict the volume of food intake, but they also change underlying neuroendocrine function.14,24 Even bariatric and metabolic surgery, however, do not halt weight gain entirely. In the Longitudinal Assessment of Bariatric Surgery study, US adults who underwent RYGB regained 3.9% (95% CI, 3.4%-4.4%) of their baseline body weight between years 3 and 7 after surgery (at 7 years, 976 patients), and individuals who underwent laparoscopic adjustable gastric banding regained 1.4% (95% CI, 0.4%-2.4%) of baseline body weight during this time (at 7 years, 322 patients).25 Findings from the Swedish Obese Subjects (SOS) study echoed these results—the mean change in body weight from baseline for patients who underwent gastric bypass, vertical banded gastroplasty, or nonadjustable or adjustable banding decreased by 23% after 2 years, whereas the mean loss in body weight for this group was only 17% after 10 years (at 10 years, 1481 patients).26
Additional hormonal drivers of obesity include both homeostatic (metabolic) and hedonic (nonmetabolic) regulators that manage food intake via a complex interplay between energetic and cognitive influences.22 Leptin, a homeostatic regulator, is a hormone that modulates food intake, energy expenditure, and glucose homeostasis under normal body conditions; in rodents, its disruption can result in severe diabetes.27-29 As obesity progresses, circulating levels of leptin increase, as well.27,28 Consistent with the hypothesis that disrupted leptin signaling partially underlies obesity, genetic deletion of the leptin receptor in hypothalamic neurons results in severe obesity and diabetes in the rodent model.29 GLP-1 also serves as a homeostatic regulator; it has receptors in the hypothalamus and hindbrain. As a patient loses weight, the GLP-1 concentration decreases, which results in decreased satiety and increased hunger.21,30,31 In the presence of these physiologic abnormalities, if the patient has ready access to an energy source, an increase in calories consumed and an increase in weight can follow. Patients who undergo bariatric surgery and still struggle to lose weight report increased subjective hunger and lower satiety levels and have lower circulating PYY and GLP-1 and higher ghrelin levels when compared with postoperative patients who achieve expected weight loss.24
Hormones and microbiota are part of the gut-brain connection, which, through both neural and endocrine routes, bidirectionally communicates nutritional composition and eating patterns between the gut and the brain.32 Metabolic and bariatric surgeries also alter the gut microbiome.24 How microbiota affect metabolic processes in the gut is unknown, but these changes occur rapidly after RYGB and SG surgery, and rodent models suggest that they are crucial in weight loss.24 More information about the gut-brain connection continues to be reported; in turn, the field of obesity treatment is rapidly expanding to seek out new methods to address this connection.32
Obesity may relate bidirectionally with sleep disturbances, which may impact hormone production.33 Sleep is implicated in insulin secretion, energy expenditure, and other weight-control pathways. A short sleep duration is associated with low levels of leptin, which decreases satiety, and high levels of ghrelin, which increases hunger.33 Moreover, people who work outside of normal daytime working schedules may experience shift work-sleep disorder, in which desired sleep-wake schedules do not align with innate sleep-wake circadian rhythms.33 Shift workers experience a greater frequency of overweight, obesity, and metabolic syndrome than do workers who have a traditional schedule.33 As such, they are more predisposed to develop pathophysiologic alterations of glucose metabolism, lipid homeostasis, and appetite regulation, and they may be susceptible to overeating, consuming poor nutrient diets, and having reduced physical activity because of daytime sleepiness.33 In addition, obesity is a well-established risk factor for such sleep disorders as obstructive sleep apnea, insomnia, and restless leg syndrome.33
Genetics
Genetic factors can contribute to the development of many diseases, including type 2 diabetes mellitus (T2D), various cancers, cardiovascular disease (CVD), and obesity.34-38 These factors also impact body mass index (BMI), a central measure of obesity that is calculated using a person’s height and weight (see Diagnosis and Treatment). In twin and family studies, genetic factors account for 40% to 70% of interindividual differences in BMI.37 The BMI of identical twins, including those separated at birth, are highly correlated. During childhood, they may be attributed to shared environment; in adulthood, however, nonshared experiences do not explain the continuing similarity.37 The heritability of BMI (BMI-H) is age-related, peaking at 20 years of age, decreasing between the ages of 20 and 55 years, and then gradually increasing again after age 55 years; the BMI-H association increases with the average level of BMI.39 The results of a 2017 study that compared the heritability of BMI across countries at various stages of economic development found that the proportionate increase in a child’s BMI, which is associated with the parent’s BMI, is approximately constant across countries and populations that are substantively different in epidemiologic terms.40 The effect is nearly doubled when both parents are considered, suggesting that obesity is directly related to the process of intergenerational transmission of the disease within families from parents to children.40
Furthermore, the results of a 2017 assessment of obesity-related genes found that the pathogenesis of obesity is related to intracellular signaling (eg, G-protein coupled receptors), cholesterol and lipid biology (eg, statin pathways), longevity, metabolism, immune mediators (eg, interleukin 7 [IL-7] signaling), feeding behaviors, cholesterol metabolic processes, glucose and cholesterol homeostasis, regulation of blood pressure, small-molecule metabolic processes, protein binding (including peptide hormone binding), hormone activity, steroid hormone receptor activity, insulin receptor binding, and enzyme binding.41 In the assessment, after obesity, the conditions most closely identified with the genes studied were noninsulin-dependent T2D, acquired metabolic disease, overnutrition, and disease of glucose metabolism.41 Most pathways and genes highly linked to obesity were related to insulin production, signaling, or related metabolic functions involving IGF1, IRS1, and INS, suggesting a physiologic link between obesity and diabetes.41 The many associations between obesity-related genes and biologic processes found in this study reinforce the physiologic complexity of this serious, chronic, relapsing, and treatable disease.2-4
Associated Health Conditions
Obesity is associated with approximately 200 health conditions, confounding its diagnosis and management.42 Massie et al examine this clinical burden in the second article of this series.10 Examples of weight-related diseases that are either caused or exacerbated by obesity include T2D, CVD, dyslipidemia, hypertension, polycystic ovary syndrome, infertility in women, hypogonadism in men, obstructive sleep apnea, asthma, osteoarthritis, mental depression, gastroesophageal reflux disease, nonalcoholic fatty liver disease, and urinary stress incontinence.15 In addition, 13 cancers and some infections, metabolic diseases, and obstetric conditions are associated with obesity.42,43 Most closely related to obesity is T2D; however, not all persons with overweight or obesity have T2D, and not all persons with T2D have overweight or obesity.15 Still, obesity can worsen existing insulin resistance and hasten the progression to diabetes.15
Although the relationship between obesity and CVD is complex, there is an association between overweight/obesity and CVD, with impact on mortality varying as a function of gender, ethnicity, age, and body fat distribution.15,44 Cancer also is prevalent in patients with obesity. In 2014, approximately 630,000 patients in the United States were diagnosed with overweight- or obesity-related cancers, capping a growth rate of 7% in the previous 9 years, when rates of cancers not related to obesity declined.43
Another consideration in persons with obesity is the likelihood of weight gain associated with medications used to treat various comorbid conditions, which can lead to treatment nonadherence and worsening of comorbidities and obesity. Commonly used medications associated with weight gain include atypical antipsychotics, antidepressants (especially tricyclic antidepressants, but also serotonin reuptake inhibitors), some anti-epileptics, certain antidiabetic agents (eg, sulfonylureas, thiazolidinediones), glucocorticoids, progestin oral contraceptives, and β-blockers. Further research into the effects of gene variations on drug response (pharmacogenomics) may help optimize the use of AOMs while minimizing medication-induced weight gain.45
Environment
Environmental factors that increase the risk for obesity development include, but are not limited to, unavailability of healthy food options, restrictive environmental access to safe physical activity, social isolation, poverty, and allostatic load.20 Amaro et al explore these social determinants of health in the fifth article of this supplement series.13 In a statement, the Endocrine Society highlighted that the coupling of modern society’s calorie-rich environment and sedentary culture with humans’ evolutionary predisposition to conserve body fat represents a condition for widespread obesity.16
Large-scale economic factors can further promote an obesogenic environment. BMI-H positively correlates both with high gross domestic product (GDP) per capita and with economic stagnation.39 Food insecurity, advertisements, prices, and presence of food deserts all correlate with childhood obesity.20 International trade agreements are another structural factor. In 1994, signing of the North American Free Trade Agreement increased the availability of soft drinks, processed foods, and meats in Mexico and preceded an obesity epidemic among the population of that country.46 Lower financial capacity, as measured with the Economic Hardship Index, is also associated with higher rates of childhood obesity, and economic hardship is correlated with race.20 Lower financial capacity can, for instance, limit access to personal transportation. Such obstacles can combine with limitations of the built environment—such as a lack of public transit and of a safe, walkable distance to grocery stores—to hinder families’ access to quality food.20
Environmental factors also influence response and access to anti-obesity treatment, including both lifestyle and medical interventions; starting as early as in childhood, these factors drive health disparities observed with obesity and other chronic diseases.47 Moreover, adverse childhood experiences, defined as traumatic or stressful events and unsafe environments that occur in or are present for children under 18 years of age, are associated with the development of obesity in adulthood. The mechanisms by which adverse childhood experiences and obesity interact are not fully understood, but they may include chronic stress, mental health issues, social disruption, socioeconomic status, sleep disorders, and stress-induced overeating along with changes in the gut microbiome.19
Diagnosis and Treatment
In 2014, the American Heart Association (AHA), the American College of Cardiology (ACC), and The Obesity Society (TOS) published a clinical practice guideline for the management of overweight and obesity in adults.48 In 2016, the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) released clinical practice guidelines that incorporated both BMI and weight-related complications into diagnosis and recommended that weight-related complications rather than a universal weight-loss target determine treatment selection.15 These guidelines represent the most current treatment guidelines for adults with obesity.
Obesity is diagnosed by a BMI measurement of at least 25 kg/m2 (patients of Asian descent, ≥ 23 kg/m2) and the presence of 1 or more weight-related complications or a BMI measurement of at least 30 kg/m2 (patients of Asian ethnicity, ≥ 25 kg/m2).15 A BMI of at least 25 kg/m2 in any patient should prompt further evaluation, but a BMI of at least 23 kg/m2 may prompt evaluation in individuals of South Asian, Southeast Asian, and East Asian ethnicity, as health risks associated with overweight and obesity are typically observed at lower BMIs in this population.15,48,49
Obesity diagnosis should include assessment of both BMI and the degree to which excess adiposity negatively affects an individual patient’s health.15 Age, sex, and body composition (eg, level of hydration, muscular composition) should also be considered.15 Excess accumulation of body fat is associated with signs and symptoms of obesity that include metabolic abnormalities, joint pain, immobility, sleep apnea, and low self-esteem.1
The AHA/ACC/TOS guideline recommends that patients with obesity lose at least 5% of their body weight; the AACE/ACE advises a loss of at least 10% of body weight to ameliorate many weight-related conditions.15,48 To meet these targets and to simultaneously manage weight-related complications, patients and providers should work together.48 A high-intensity lifestyle intervention program (≥ 14 sessions in 6 months) is recommended as the foundation of treatment for overweight or obesity.15,48
Pharmacotherapy or AOMs can be used with lifestyle modifications to produce greater and more sustained weight loss when compared with lifestyle modifications alone.15,48 The AACE/ACE guidelines recommend that this combination be considered for all individuals with a BMI of at least 27 kg/m2 if lifestyle therapy fails to halt weight gain and is recommended for individuals with a BMI of at least 27 kg/m2 and at least 1 severe weight-related condition.15
For patients with a BMI of at least 40 kg/m2 and for those with at least 1 severe weight-related condition and a BMI of at least 35 kg/m2, metabolic and bariatric surgery should also be considered.15 In patients with obesity, this surgery has led to the greatest weight loss, continuance of weight loss, and amelioration of weight-related complications and to decreased mortality.26,48
Weight loss from lifestyle interventions, medical therapy, and bariatric surgery can effectively treat T2D and hypertension and substantially reduce early mortality, progression of T2D, and risk of CVD and stroke. However, for most patients, lifestyle interventions alone cannot reverse hormonal and metabolic abnormalities that come after weight loss; multiple interventions may be required for patients with obesity to sustain medically meaningful weight loss for the clinically significant period of 12 months or more.1,50 In a November 2022 guideline, the American Gastroenterological Association (AGA) emphasized the particular need for medical therapy. In evaluating safety and total body weight loss reported in randomized controlled trials across FDA-approved pharmacologic agents, the AGA guideline authors strongly recommend that an AOM be added to lifestyle intervention for all adults with obesity who have had insufficient response to lifestyle intervention alone.50 They add that these agents generally need to be used chronically because of the chronic nature of obesity.50 In the third article of this supplement series, Cornier explores the clinical guidelines for both diagnosis and treatment in greater detail.11
The AACE/ACE guidelines do not recommend weight-loss therapy only to prevent cardiovascular events or prolong life in patients with CVD, but they underscore the need for further investigation.15 Since publication of the guidelines in 2016, such research has taken place. For example, in an SOS study of the effects of bariatric surgery on mortality and life expectancy in patients with obesity, patients who underwent surgery (n = 2007) were 30% less likely to die from CVD than were patients in the control group (n = 2037; HR, 0.70 [95% CI, 0.57-0.85]).51
A lower rate of obesity-related cancer in patients noted among patients who underwent bariatric surgery underscores the relationship between obesity and cancer. A large, retrospective, observational, matched cohort study followed 30,318 patients with obesity in the United States; after 10 years, among 5510 patients, individuals who underwent RYGB or SG (n = 939) were 32% (95% CI, 13%-47%) less likely to have obesity-related cancer than were those who received usual care (n = 4571).52
Growing Prevalence and Burden of Obesity
Following global trends, the prevalence of this serious medical condition in adults in the United States increased markedly during the 21st century. According to data from the National Health and Nutrition Examination Survey (NHANES), the age-adjusted prevalence of overweight and obesity in US adults rose from an estimated 56% in 1988-1994 to approximately 73.1% in 2017-2018.53 Data gathered in recent years reinforce this trend: the proportion of US adults with obesity increased from 30.5% in 1999-2000 to 39.6% in 2016 and then to 42.4% in 2018.53 From 1999-2000 to 2017-2018, the prevalence of severe obesity (BMI ≥ 40 kg/m2) increased from 4.7% to 9.2% (Figure).54 Weight gain, which propels obesity, also increases with age. A 2022 study using 2011-2018 NHANES data on 13,802 US adults found that the mean 10-year weight gain was 6.6% (SE, ± 0.2%) of initial body weight.55
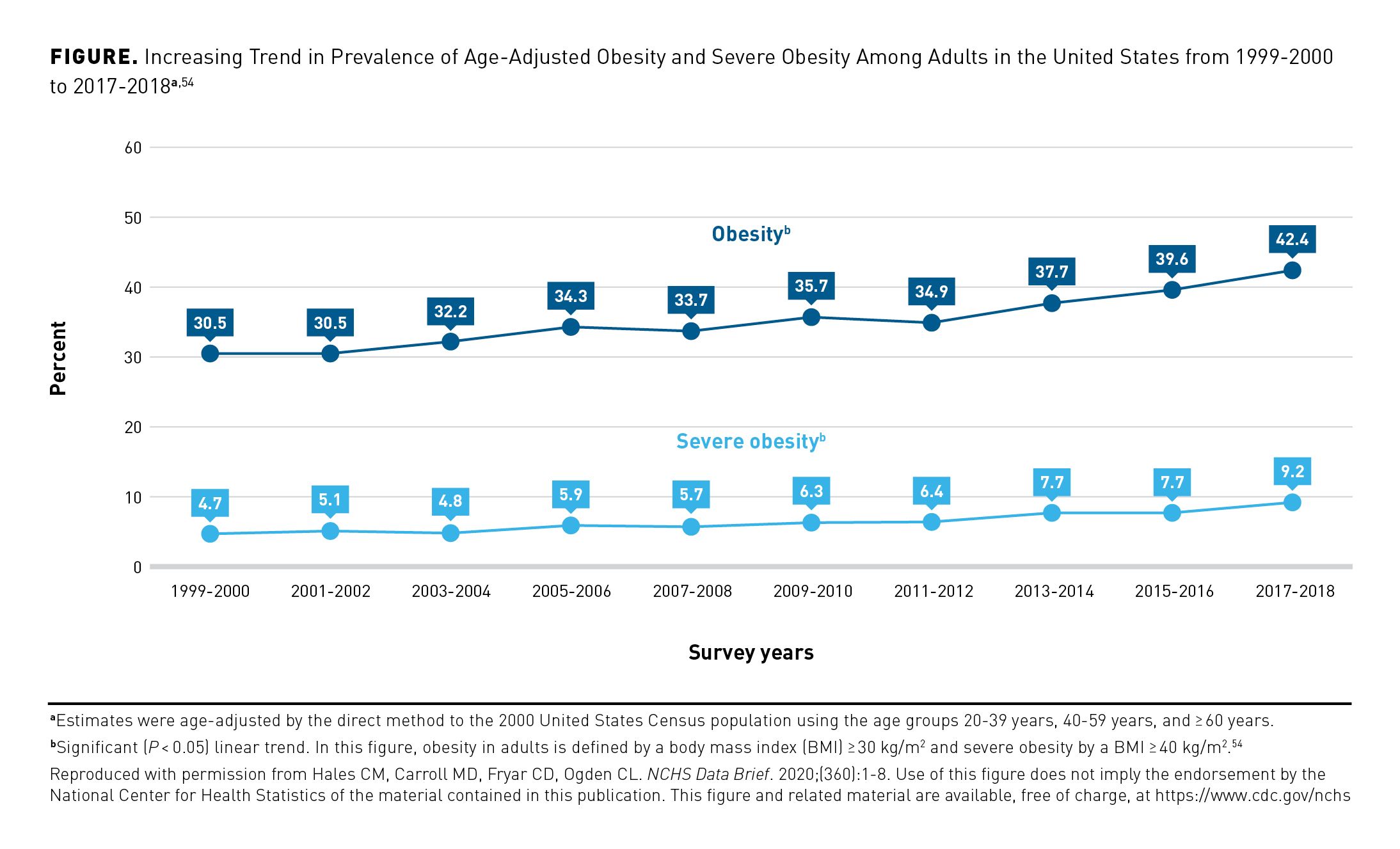
Trends by patient gender, age, race, and ethnicity show clearly where overweight and obesity most affect American adults.54 In particular, patterns for severe obesity illustrate demographic differences. In 2017-2018, women had a significantly higher prevalence of severe obesity (11.5%) than did men (6.9%).54 Adults aged 40 to 59 years (11.5%) had the highest prevalence of severe obesity; those aged 20 to 39 years had the next-highest prevalence (9.1%); and adults 60 years and over had the lowest (5.8%).54 Non-Hispanic Black adults experienced the highest prevalence of severe obesity (13.8%); non-Hispanic White adults, Hispanic adults, and non-Hispanic Asian adults experienced lower prevalence (9.3%, 7.9%, and 2.0%, respectively).54 Trends in weight gain reflect some of these demographic differences. A 2022 study of US adults that used NHANES data reported that women gained 2.4 times more weight over a 10-year period than did men (when considering percentage weight gain); non-Hispanic Black adults had the largest weight gain during this period, and non-Hispanic Asian patients had the smallest weight gain.55
The COVID-19 pandemic exacerbated the obesity pandemic. Infection with SARS-CoV-2 is associated with persistent myocardial injury, worsening control of blood glucose level in T2D, reduced ability to be physically active, increased mental depression and anxiety, and effects on food consumption and purchasing bias; all of these may affect weight and weight control activities.6 In turn, obesity increases the risk for mortality, severe morbidity, and lasting COVID-19–related complications among those infected with SARS-CoV-2.56,57 The growing prevalence of overweight and obesity in the United States and the association of these medical problems with increased morbidity and mortality underscore the need for clinical intervention.
Clinical, Economic, and Humanistic Burden
Massie et al explore fully the clinical, economic, and humanistic burden of obesity in the second article of this supplement series; however, some highlights are discussed here. The impact of obesity has an enormous effect on health care spending. The direct health care costs of chronic diseases linked to the risk factors of overweight and obesity in 2016 amounted to $480.7 billion, and indirect costs from lost economic productivity accounted for another $1.24 trillion. In total, costs associated with chronic diseases related to obesity and overweight totaled $1.72 trillion, or 9.3% of that year’s GDP.9,10
Obesity is associated with a substantial burden apart from physical comorbidities. This burden includes mental disorders, mood disorders, anxiety, and major depression.58,59 In both men and women, a bidirectional relationship exists between mental depression and body weight. In people with obesity, symptoms of depression may be caused by their negative body image. Obesity is also a risk factor for a variety of comorbidities (eg, T2D, CVD) associated with the development of depression. In turn, modified eating patterns or reduced physical activity related to depression may impact body weight.59 Emotions, stress, and cultural food cues often prompt disordered eating, which occurs frequently in patients with obesity.58 Further, obesity negatively affects self-esteem, body image, quality of life, and level of anxiety in social situations, and it can increase exposure to social, educational, employment, and health care stigma and discrimination.58
Advancements in National Policy Due to Medical Acceptance
In designating obesity as a disease, the AMA joined several major medical organizations, including the AACE, the ACC, the Endocrine Society, the American Society for Reproductive Medicine, and the American College of Surgeons.1,15 As Amaro et al detail in the fifth article of this supplement series, patient, provider, and employer stigma and misperceptions surrounding obesity and lack of treatment coverage pose significant barriers to treatment.13 However, several key advancements in policy led to the AMA’s 2013 designation of obesity as a disease, which, in itself, led to new and proposed policies supporting those who have obesity or overweight.17,60 The Treat and Reduce Obesity Act is proposed legislation calling for expansion of Medicare coverage of intensive behavioral therapy for people with obesity and coverage of medications used for obesity or weight loss management under Medicare’s prescription drug benefit.60 Additional strides have been made in reducing social stigma and in increasing legal protections of persons with obesity and overweight. The Equal Employment Opportunity Commission has won court cases involving obesity as a protected disability under the Americans with Disabilities Act, and employment law firms have printed opinions warning employers that discrimination suits could be brought to court for obesity discrimination.17 These advances show the positive effect of classifying obesity as a chronic disease, yet structural barriers to obesity therapy remain. For example, the US Department of Health and Human Services Healthy People 2030 program acknowledges the need for public health interventions and culturally appropriate programs and policies to foster healthy eating, physical activity, and maintenance of a healthy weight.61 Strong support from the AMA may bolster national policy. For instance, the AMA recently declared that it would lead a comprehensive initiative to advance the study, prevention, and treatment of obesity and to involve state and national medical societies in this effort.62
Conclusions
That obesity is a serious, chronic, relapsing, and treatable disease associated with multiple comorbidities and requiring long-term medical management underscores the need for multimodal care strategies.1,63
Since development of the 2 comprehensive, US-based guidelines from the AHA/ACC/TOS and AACE/ACE, the management of obesity has changed considerably. Over the few years after critical questions were chosen for the AHA/ACC/TOS guideline, the FDA approved 4 AOMs for the long-term treatment of this disease.15,48,64-67 Since publication of the AACE/ACE guidelines, another long-term AOM was approved, an AOM was withdrawn from the market, and several procedures and devices for weight loss were recommended by the American Society for Metabolic and Bariatric Surgery (ASMBS) or became regulated by the FDA.64,68-70 Moreover, updated clinical practice recommendations have been provided by organizations such as the Obesity Medicine Association, which updates the Obesity Algorithm and published its inaugural issue of a peer-reviewed, evidence-based journal for clinicians in March 2022.4,71 Further, the ASMBS and the International Federation for the Surgery of Obesity and Metabolic Disorders issued a joint statement in November 2022 that broadens the population for whom it recommends metabolic and bariatric surgery.72 A need exists for new collaborative guidelines that incorporate recent research and that are inclusive of guidance on current therapies.
Regardless, evidence has shown that lifestyle intervention alone may not be enough to achieve and maintain medically meaningful weight loss for the clinically significant period of 12 months or more.1,50 FDA-approved treatments for chronic weight management have helped affected persons to achieve and sustain significant weight loss and other health benefits.15 In particular, pharmacotherapy and a multifaceted approach that involves intensive lifestyle/behavioral therapy is effective, and this strategy should be considered for eligible individuals.15,73
Acknowledgements
This peer-reviewed supplement was funded by Novo Nordisk Inc. The authors acknowledge the professional medical writing support from Clinical Communications, a division of MJH Life Sciences®, Cranbury, NJ, which received funding support from Novo Nordisk Inc, Plainsboro, NJ. Novo Nordisk Inc. provided scientific and medical accuracy review of this publication.
Author Affiliations: Clinical Nutrition Center and University of Colorado Anschutz Medical Campus (EL), Greenwood Village and Denver, Colorado; MedExpress/Optum Health Services (SOP), Minneapolis, MN.
Funding Source: This supplement was supported by Novo Nordisk.
Author Disclosures: Dr Lazarus reports being a board member for the Obesity Medicine Association and reports serving as a paid consultant or paid advisory board member for Currax Pharmaceuticals and Nestle Health Sciences. He also reports receiving honoraria from Currax Pharmaceuticals, Nestle Health Sciences, Novo Nordisk, and the Obesity Medicine Association and lecture fees from Novo Nordisk and Currax Pharmaceuticals. Dr Ortiz-Pujols reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (EL, SOP); drafting of the manuscript (EL, SOP); and critical revision of the manuscript for important intellectual content (EL, SOP).
Address Correspondence to: Ethan Lazarus, MD, Clinical Nutrition Center, 5995 Greenwood Plaza Blvd, Ste 150, Greenwood Village, CO 80111. Email: ethanlazarus@gmail.com
References
- American Medical Association House of Delegates, 2013. Recognition of obesity as a disease. Resolution 420 (A-13). May 16, 2013. Chicago, USA. NPR. Accessed July 22, 2022. https://media.npr.org/documents/2013/jun/ama-resolution-obesity.pdf
- Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obesity (Silver Spring). 2018;26(1):61-69. doi:10.1002/oby.22054
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization. 2000. Accessed July 22, 2022. https://apps.who.int/iris/bitstream/handle/10665/42330/WHO_TRS_894.pdf?sequence=1&isAllowed=y
- Lazarus E. OMA president’s perspective: a new journal will help us overcome obesity. Obesity Pillars. 2022;1(1):1-2. doi:10.1016/j.obpill.2021.100002
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
- Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected – obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135-149. doi:10.1038/s41574-020-00462-1
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766-781. doi:10.1016/S0140-6736(14)60460-8
- Obesity and overweight. World Health Organization. June 9, 2021. Accessed July 22, 2022. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- Waters H, Graf M. America’s obesity crisis: the health and economic costs of excess weight. Milken Institute. October 2018. Accessed July 22, 2022. https://milkeninstitute.org/sites/default/files/reports-pdf/Mi-Americas-Obesity-Crisis-WEB_2.pdf
- Massie DC, Amaro A, Kaplan M. Patient well-being and the clinical and economic burdens associated with obesity in the United States. Am J Manag Care. 2022;28(suppl 15):S279-S287. doi:10.37765/ajmc.2022.89291
- Cornier MA. a review of current guidelines for the treatment of obesity. Am J Manag Care. 2022;28(suppl 15):S288-S296. doi:10.37765/ajmc.2022.89292
- Kyrillos J. Semaglutide 2.4-mg injection as a novel approach for chronic weight management. Am J Manag Care. 2022;28(suppl 15):S297-S306. doi:10.37765/ajmc.2022.89293
- Amaro A, Kaplan M, Massie DC. Managed care considerations of weight management interventions for obesity. Am J Manag Care. 2022;28(suppl 15):S307-S318. doi:10.37765/ajmc.2022.89294
- Farias MM, Cuevas AM, Rodriguez F. Set-point theory and obesity. Metab Syndr Relat Disord. 2011;9(2):85-89. doi:10.1089/met.2010.0090
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi:10.4158/EP161365.GL
- Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38(4):267-296. doi:10.1210/er.2017-00111
- Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin North Am. 2016;45(3):511-520. doi:10.1016/j.ecl.2016.04.004
- Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673-689. doi:10.1007/s40273-014-0243-x
- Schlauch KA, Read RW, Neveux I, Lipp B, Slonim A, Grzymski JJ. The impact of ACEs on BMI: an investigation of the genotype-environment effects of BMI. Front Genet. 2022;13:816660. doi:10.3389/fgene.2022.816660
- Vargas CM, Stines EM, Granado HS. Health-equity issues related to childhood obesity: a scoping review. J Public Health Dent. 2017;77(suppl 1):S32-S42. doi:10.1111/jphd.12233
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1160. doi:10.1056/NEJMoa1105816
- Münzberg H, Laque A, Yu S, Rezai-Zadeh K, Berthoud HR. Appetite and body weight regulation after bariatric surgery. Obes Rev. 2015;16(suppl 1):77-90. doi:10.1111/obr.12258
- Holst JJ, Rosenkilde MM. GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J Clin Endocrinol Metab. 2020;105(8):e2710-e2716. doi:10.1210/clinem/dgaa327
- Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest. 2019;42(2):117-128. doi:10.1007/s40618-018-0892-2
- Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427-434. doi:10.1001/jamasurg.2017.5025
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56-65. doi:10.1001/jama.2011.1914
- Zhao S, Kusminski CM, Elmquist JK, Scherer PE. Leptin: less is more. Diabetes. 2020;69(5):823-829. doi:10.2337/dbi19-0018
- Pan WW, Myers MG Jr. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci. 2018;19(2):95-105. doi:10.1038/nrn.2017.168
- Xu J, Bartolome CL, Low CS, et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature. 2018;556(7702):505-509. doi:10.1038/s41586-018-0049-7
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515-520. doi:10.1172/JCI990
- Romaní-Pérez M, Bullich-Vilarrubias C, López-Almela I, Liebana-Garcia R, Olivares M, Sanz Y. The microbiota and the gut-brain axis in controlling food intake and energy homeostasis. Int J Mol Sci. 2021;22(11):5830. doi:10.3390/ijms22115830
- Rodrigues GD, Fiorelli EM, Furlan L, Montano N, Tobaldini E. Obesity and sleep disturbances: the “chicken or the egg” question. Eur J Intern Med. 2021;92:11-16. doi:10.1016/j.ejim.2021.04.017
- Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505-1513. doi:10.1038/s41588-018-0241-6
- LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407-415. doi:10.1038/s41436-019-0633-8
- Inouye M, Abraham G, Nelson CP, et al; UK Biobank CardioMetabolic Consortium CHD Working Group. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72(16):1883-1893. doi:10.1016/j.jacc.2018.07.079
- Hebebrand J, Hinney A, Knoll N, Volckmar AL, Scherag A. Molecular genetic aspects of weight regulation. Dtsch Arztebl Int. 2013;110(19):338-344. doi:10.3238/arztebl.2013.0338
- Dubois L, Ohm Kyvik K, Girard M, et al. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: an international study of over 12,000 twin pairs. PLoS One. 2012;7(2):e30153. doi:10.1371/journal.pone.0030153
- Min J, Chiu DT, Wang Y. Variation in the heritability of body mass index based on diverse twin studies: a systematic review. Obes Rev. 2013;14(11):871-882. doi:10.1111/obr.12065
- Dolton P, Xiao M. The intergenerational transmission of body mass index across countries. Econ Hum Biol. 2017;24:140-152. doi:10.1016/j.ehb.2016.11.005
- Gabrielli AP, Manzardo AM, Butler MG. Exploring genetic susceptibility to obesity through genome functional pathway analysis. Obesity (Silver Spring). 2017;25(6):1136-1143. doi:10.1002/oby.21847
- Yuen M, Earle R, Kadambi N, et al. A systematic review and evaluation of current evidence reveals 195 obesity-associated disorders (OBAD). Poster presented at: ObesityWeek 2016; October 31 to November 4, 2016; New Orleans, LA. Poster T-P-3166.
- Cancers associated with overweight and obesity make up 40 percent of cancers diagnosed in the United States. Press release. Centers for Disease Control and Prevention. October 3, 2017. Accessed July 22, 2022. https://www.cdc.gov/media/releases/2017/p1003-vs-cancer-obesity.html
- Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89-100. doi:10.2147/VHRM.S168946
- Singh S, Ricardo-Silgado ML, Bielinski SJ, Acosta A. Pharmacogenomics of medication-induced weight gain and antiobesity medications. Obesity (Silver Spring). 2021;29(2):265-273. doi:10.1002/oby.23068
- Clark SE, Hawkes C, Murphy SM, Hansen-Kuhn KA, Wallinga D. Exporting obesity: US farm and trade policy and the transformation of the Mexican consumer food environment. Int J Occup Environ Health. 2012;18(1):53-65. doi:10.1179/1077352512Z.0000000007
- Byrd AS, Toth AT, Stanford FC. Racial disparities in obesity treatment. Curr Obes Rep. 2018;7(2):130-138. doi:10.1007/s13679-018-0301-3
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25;pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
- Bays HE, Shrestha A, Niranjan V, Khanna M, Kambhamettu L. Obesity pillars roundtable: obesity and South Asians. Obesity Pillars. 2022;1(10006). doi:10.1016/j.obpill.2021.100006
- Grunvald E, Shah R, Hernaez R, et al; AGA Clinical Guidelines Committee. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022;163(5):1198-1225. doi:10.1053/j.gastro.2022.08.045
- Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383(16):1535-1543. doi:10.1056/NEJMoa2002449
- Aminian A, Wilson R, Al-Kurd A, et al. Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA. 2022;327(24):2423-2433. doi:10.1001/jama.2022.9009
- Fryar CD, Carroll MD, Afful J; Division of Health and Nutrition Examination Surveys. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2017-2018. Centers for Disease Control and Prevention; National Centers for Health Statistics. December 2020. Revised January 29, 2021. Accessed July 22, 2022. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/overweight-obesity-adults-H.pdf
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Tucker LA, Parker K. 10-Year weight gain in 13,802 US adults: the role of age, sex, and race. J Obes. 2022;2022(360):1-10. doi:10.1155/2022/7652408
- Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death – United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):355-361. doi:10.15585/mmwr.mm7010e4
- Aminian A, Bena J, Pantalone KM, Burguera B. Association of obesity with postacute sequelae of COVID-19. Diabetes Obes Metab. 2021;23(9):2183-2188. doi:10.1111/dom.14454
- Sarwer DB, Polonsky HM. The psychosocial burden of obesity. Endocrinol Metab Clin North Am. 2016;45(3):677-688. doi:10.1016/j.ecl.2016.04.016
- Zhang J. The bidirectional relationship between body weight and depression across gender: a simultaneous equation approach. Int J Environ Res Public Health. 2021;18(14):7673. doi:10.3390/ijerph18147673
- Treat and Reduce Obesity Act of 2021, S.596, 117th Cong. (2021-2022). Congress.gov. March 4, 2021. Accessed July 22, 2022. https://www.congress.gov/bill/117th-congress/senate-bill/596
- Overweight and obesity. Healthy People 2030. US Department of Health and Human Services. Accessed July 22, 2022. https://health.gov/healthypeople/objectives-and-data/browse-objectives/overweight-and-obesity
- Addressing obesity D-440.954. American Medical Association. 2022. Accessed November 23, 2022. https://policysearch.ama-assn.org/policyfinder/detail/obesity?uri=%2FAMADoc%2Fdirectives.xml-0-1498.xml
- Bray G, Kim K, Wilding JOH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Belviq, Belviq XR (lorcaserin) by Eisai: Drug safety communication – FDA requests withdrawal of weight-loss drug. Press release. FDA. February 13, 2020. Accessed July 22, 2022. https://www.fda.gov/safety/medical-product-safety-information/belviq-belviq-xr-lorcaserin-eisai-drug-safety-communication-fda-requests-withdrawal-weight-loss-drug
- Qsymia. Prescribing information. VIVUS; 2022. Accessed July 22, 2022. https://hcp.qsymia.com/include/media/pdf/prescribing-information.pdf
- Contrave. Prescribing information. Currax; 2021. Accessed July 22, 2022. https://www.contravehcp.com/wp-content/uploads/Contrave_PI_MedGuide.pdf
- Saxenda®. Prescribing information. Novo Nordisk; 2022. Accessed July 22, 2022. https://www.novo-pi.com/saxenda.pdf
- Wegovy®. Prescribing Information. Novo Nordisk; June 2021. Accessed July 22, 2022. https://www.novo-pi.com/wegovy.pdf
- FDA approves new drug treatment for chronic weight management, first since 2014. Press release. FDA. June 4, 2021. Accessed July 22, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- ASMBS endorsed procedures and FDA approved devices. American Society for Metabolic & Bariatric Surgery. Updated May 11, 2022. Accessed July 13, 2022. https://asmbs.org/resources/endorsed-procedures-and-devices
- Obesity Algorithm. Obesity Medicine Association. 2021. Accessed November 23, 2022. https://obesitymedicine.org/obesity-algorithm/
- Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022. Published online October 18, 2022. doi:10.1016/j.soard.2022.08.013
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415

