- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Improving Outcomes and Mitigating Costs Associated With CAR T-Cell Therapy
Abstract
Since the historic approval of tisagenlecleucel for the treatment of B-cell acute lymphoblastic leukemia in 2017, chimeric antigen receptor (CAR) T-cell therapies have altered the treatment paradigm for hematologic malignancies. Five CAR T-cell products are now approved by the US Food and Drug Administration for a growing number of cancer indications and a global market worth billions is anticipated in the next 5 years. While uptake of CAR T-cell therapy is rapidly ramping up, there remain significant barriers to effective implementation and patient access, not least their price tag and substantial ancillary costs of care. CAR T-cell therapies currently have the potential to be cost-effective; however, improved safety and efficacy, outpatient administration, and a streamlined manufacturing process could make them even more so. In the meantime, payers and providers are tasked with facing the logistical complexities of CAR T-cell therapy and developing new payment and reimbursement strategies to ensure value-based care and optimal access today.
Am J Manag Care. 2021;27(13):S253-S261. https://doi.org/10.37765/ajmc.2021.88737
Introduction
Chimeric antigen receptor (CAR) T-cell therapies represent a paradigm shift for the treatment of several previously incurable refractory hematologic malignancies. Their use is rapidly expanding as more become approved by the FDA. In 2018, just 2 CAR T-cell therapies were commercially available: axicabtagene ciloleucel for the treatment of relapsed/refractory large B-cell lymphoma (LBCL), and tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) and adult patients with relapsed or refractory LBCL.1,2 Axicabtagene ciloleucel is now also approved for the treatment of patients with relapsed or refractory follicular lymphoma.3
In the past 2 years, these CAR T-cell products have been joined by 3 others. In July 2020, brexucabtagene autoleucel became the first approved product for relapsed or refractory mantle cell lymphoma (MCL), while lisocabtagene maraleucel was approved for the treatment of LBCL in February 2021.3,4 All of the aforementioned CAR T-cell products target the CD19 antigen, but in March 2021, idecabtagene vicleucel made history as the first B-cell maturation antigen (BCMA)-targeting CAR T-cell therapy approved for the treatment of adult patients with relapsed/refractory multiple myeloma.3 Table 15-10 summarizes the currently approved CAR T-cell products.
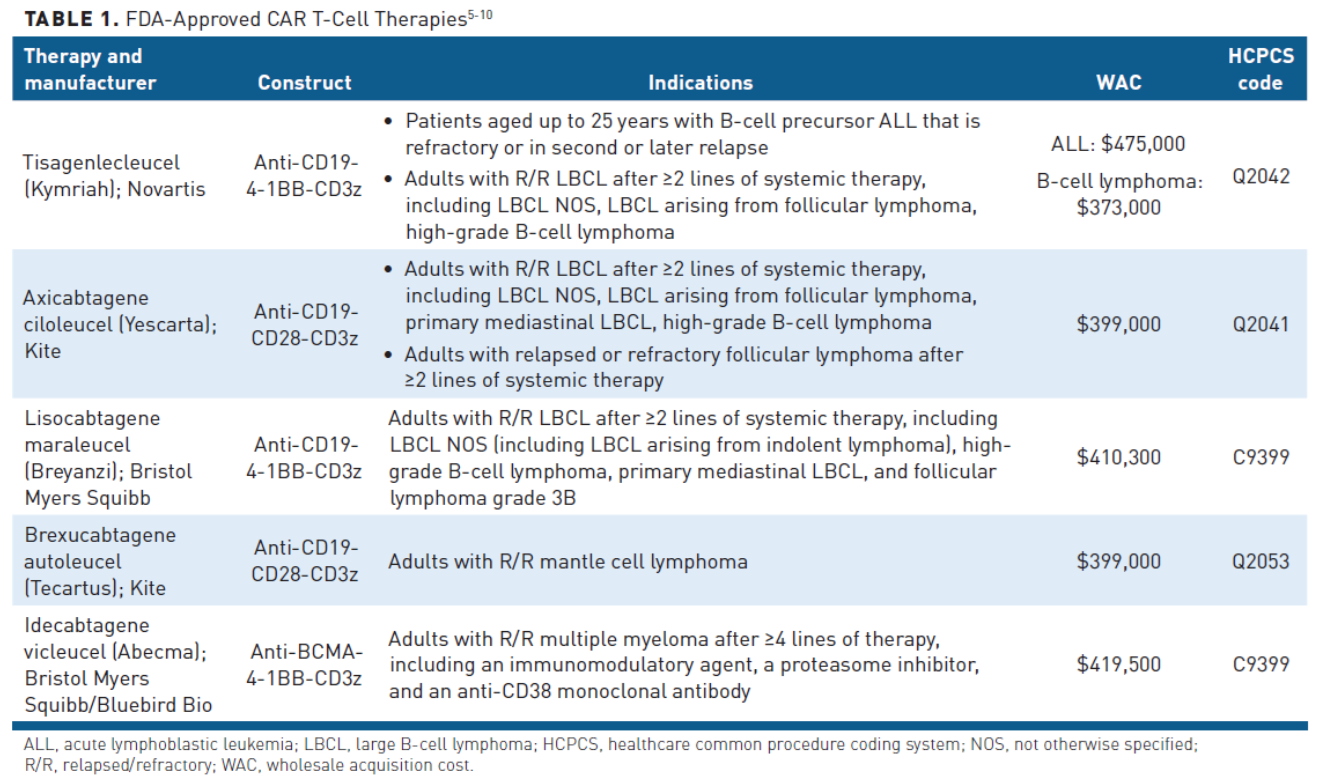
The field continues to rapidly evolve, with the manufacturers of brexucabtagene autoleucel and a second BCMA-targeted CAR T-cell therapy, ciltacabtagene autoleucel, seeking approval of their products for the treatment of relapsed or refractory ALL with a Prescription Drug User Fee Act (PDUFA) date of October 1, 2021 and multiple myeloma with a PDUFA date of November 29, 2021, respectively.11,12 Many more CAR T-cell therapies are in development, with more than 600 clinical trials underway globally.13
Uptake of CAR T-cell therapy has been slow, largely limited to large transplant centers where clinical trials were performed. However, in recent years, it has been rapidly expanding; according to an analysis of the Medicare fee-for-service population, the number of CAR T-cell claims are doubling every 6 months.14 This is a trend that looks set to continue, with the rush to begin offering CAR T-cell therapy to patients with multiple myeloma, the shift to an outpatient setting, growing experience in managing the logistics and complexities of CAR T-cell therapy, and improving reimbursement mechanisms. Multiple different market research companies have performed analyses of the current and future trends in the CAR T-cell market. While their predicted growth estimates vary, all anticipate a global market of many billions of dollars within the next 5 years.15-17 There is an urgent need for payers and healthcare systems to finetune the logistics of CAR T-cell therapy as they gain broader traction.
Direct Costs
In spite of the growing CAR T-cell market, there are still significant barriers to optimal uptake and effective implementation. The cost of CAR T-cell therapy is one of the biggest challenges, with financial implications for patients, payers, and providers. Drug acquisition is the largest component of the cost of CAR T-cell therapy, with list prices ranging from $373,000 to $475,000 depending on the specific drug and indication.18 However, the costs associated with CAR T-cell therapies are not limited to acquisition costs alone and other ancillary elements of care have a significant impact on healthcare expenditures (Table 219).
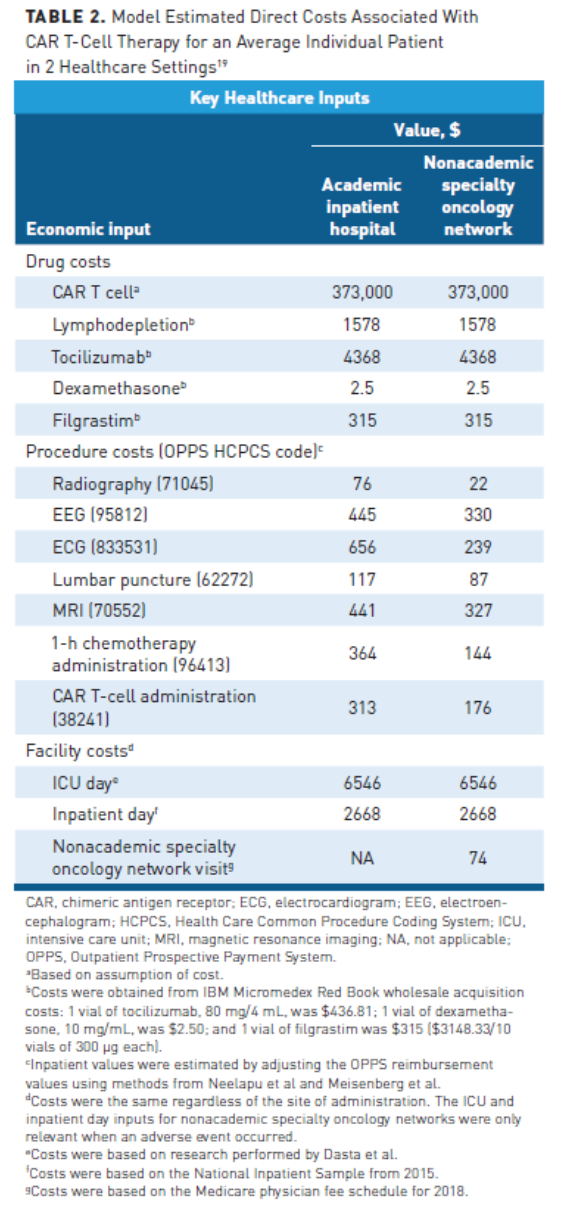
CAR T-cell therapies are currently administered at more than 100 medical centers in the United States, primarily in the inpatient setting, although there is an increasing trend toward outpatient administration.19-21 Next to drug acquisition, hospital services are the most significant driver of cost for CAR T-cell therapies, particularly for patients who experience toxicities.19 In 2 separate analyses of the cost outcomes of patients undergoing CAR T-cell therapy identified from hospital administrative data, one in pediatric and young adult patients with ALL, the other in adults with B-cell lymphoma, the majority treated in the inpatient setting, the median total cost of hospitalization for CAR T-cell therapy exceeded $300,000 and was closer to $400,000 in pediatric and young adult patients.22 The primary cause of hospitalization for outpatients or transfer to an intensive care unit (ICU) as an inpatient is cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS). According to a multicenter historical cohort study of 111 patients treated with CAR T-cell therapy at 7 medical centers in the United States who were admitted to the ICU, CRS was the primary cause of admission in 41% of cases, with overlapping CRS and ICANS present in 60% of patients.23
Beyond the CAR T-cell therapy itself, there are numerous other associated drug costs that contribute to the total cost of treatment. Among pre-infusion costs are lymphodepleting chemotherapy and potential bridging therapy. With regard to the latter, the average time from apheresis until CAR T-cell infusion varies according to the specific product (the median turnaround times range from 15 days with brexucabtagene autoleucel to 33 days with idecabtagene vicleucel) but with administrative delays or manufacturing issues this period may be longer.8,9 Patients with progressive symptomatic disease may require some kind of treatment to support them during that time, although there are still many outstanding questions about the optimal type of bridging therapy.24
During and after CAR T-cell therapy, the majority of other drug-related direct costs are incurred as a result of either prophylaxis or treatment of associated toxicities. CRS and ICANS are the most common adverse effects (AEs) associated with CAR T-cell therapy, and patients are also at increased risk of infection for several reasons. As such, commonly used drugs include seizure prophylaxis (eg, levetiracetam), bacterial, viral, and fungal infection prophylaxis and treatment, growth factors such as filgrastim as standard of care during periods of neutropenia, and the interleukin (IL)-6 receptor antagonists tocilizumab and siltuximab, as well as corticosteroids, for the management of CRS. The FDA Risk Evaluation and Mitigation Strategy (REMS) for CAR T-cell therapy requires that the pharmacy stock a minimum of 2 doses of tocilizumab for each patient.21 B-cell aplasia and hypogammaglobulinemia can also result from depletion of healthy B cells following CAR T-cell therapy and may be associated with long-term remission and CAR T-cell persistence. B-cell aplasia can be effectively managed with immunoglobulin replacement therapy, but monthly doses of intravenous immunoglobulin can cost as much as $5000 to 10,000 per infusion.25
Several nondrug-related costs are associated with CAR T-cell therapy. Although they are significantly less than drug-related costs, nondrug costs are still substantial, with an estimated range of $30,000 to $56,000 from current experience with tisagenlecleucel and axicabtagene ciloleucel.26 Generation of CAR T-cell therapies begins with the collection of the patient’s blood and isolation of the lymphocytes contained therein via the process of leukapheresis. The Centers for Medicare & Medicaid Services-assigned HCPCS codes for each of the various CAR T-cell products contain the drug name as well as wording stating “including leukapheresis and dose preparation procedures.”27,28 There have been some objections from provider groups to bundling up the costs of leukapheresis, storage, and handling into the cost of the drug. Many centers are likely to bill for these items separately and commercial payers will be left to determine how they choose to reimburse those items.29,30 Other nondrug-related costs include laboratory costs, organ procurement, revenue codes, and blood.22
Total Cost of Care
Beyond the direct costs of CAR T-cell therapy, there is another economic burden that is often overlooked. This includes the numerous out-of-pocket expenses for patients and their caregivers, such as the need for accommodation and transportation. Per the REMS requirements, patients should remain within a 2-hour radius of the medical center for at least 4 weeks after CAR T-cell therapy and driving within the first 8 weeks post-therapy is restricted. Although the number of centers performing CAR T-cell therapy is expanding, patients and their caregivers may still have to travel long distances if they do not live in proximity to such institutions. There is also a significant potential for loss of earnings during and after treatment for both patient and caregiver.31
There are also hidden costs to the institutions performing CAR T-cell therapy, including the need to hire additional specialized staff and/or train existing staff, gain REMS certification, and educate patients and caregivers. An open question for institutions is whether to treat CAR T-cell therapies as drugs, which determines whether they are processed by the pharmacy or the cellular therapy department. In many cases, institutions are choosing a mixture of both, with the pharmacy involved in billing, registering, verifying, and labeling the product. Indirect costs can present a particular challenge for budgeting and strategizing the introduction of CAR T-cell therapy to an institution.31
Gaining a fuller understanding of the total cost of care for CAR T-cell therapy is essential to effective management of this high-cost therapy to reduce the economic burden on payers, providers, and patients. The CAR T-cell therapy field is still in its infancy and available real-world cost data are limited, but several studies have estimated total costs of care to be between $450,000 and $480,000, with one study estimating total costs to far exceed $500,000 in patients with severe CRS (grade ≥3).19,26 However, there is growing appreciation in the field that the actual total cost of care may be closer to $1 million or even $2 million.32,33 The American Society for Transplantation and Cellular Therapy has advised hospitals administering CAR T-cell therapy to bill 4 to 5 times the price of the drug, or $2 million to $2.5 million, to avoid losing money.33
Value and Affordability
Given the staggering cost, determining the value of CAR T-cell therapy and how that compares to alternative therapies has important implications. Several model-based, cost-effectiveness analyses have compared tisagenlecleucel and axicabtagene ciloleucel with standard of care in the US healthcare system. These studies used US-based costs for inpatient CAR T-cell administration and possible subsequent treatments, as well as the costs of disease monitoring and treatment of AEs over the short and long term, and reported the potential health outcomes gained (commonly quantified as quality-adjusted life-years [QALYs], a function of length and quality of life) per dollar spent.34
In pediatric and young adult patients with B-cell ALL, tisagenlecleucel was compared with clofarabine monotherapy, clofarabine combination therapy, or blinatumomab over a patient lifetime horizon. Across these studies, tisagenlecleucel was more costly than standard of care, but was associated with greater health gains. Cost-effectiveness estimates ranged from $37,000/QALY to $184,000/QALY, depending on model assumptions relating to cost and long-term survival.35-37 By comparing the cost and QALYs associated with CAR T-cell and standard-of-care therapies, an incremental cost-effectiveness ratio can be derived; when 40% of patients are presumed to show greater than 5-year progression-free survival, tisagenlecleucel demonstrates an incremental cost-effectiveness ratio of $168,000/QALY in this setting.34
In patients with B-cell lymphoma, the cost-effectiveness of axicabtagene ciloleucel compared with chemotherapy was modeled in 2 different studies. Again, the costs for CAR T-cell therapy were higher, but so were QALYs gained. Cost-effectiveness estimates ranged from $58,000 to $289,000/QALY. In a separate study, tisagenlecleucel was shown to have similar cost-effectiveness to axicabtagene ciloleucel in the setting of B-cell lymphoma. A conservative estimate of cost-effectiveness was $223,000/QALY and an optimistic estimate was $168,000/QALY for tisagenlecleucel in this cancer type.38,39
Costs per QALY can be compared with a predetermined willingness to pay (WTP) threshold to help payers determine the value of funding a therapy (Table 334). The World Health Organization suggests a WTP of 3 times the gross domestic product per capita per QALY, which would represent $180,000/QALY in the United States currently, but a range of $100,000 to $150,000 is the most universally accepted in the United States. To help inform coverage decisions, the Institute for Clinical and Economic Review (ICER) conducts value assessments of newly approved therapies and most payers will default to this assessment if they formally consider cost-effectiveness in their coverage decisions.34 ICER panel determined that cost-effectiveness estimates were below or within WTP thresholds in the United States over a lifetime horizon and that tisagenlecleucel for the treatment of ALL was an intermediate long-term value for the money, while axicabtagene ciloleucel for B-cell lymphoma was low-to-intermediate long-term value.40 At the time of this assessment, tisagenlecleucel was not yet approved for the treatment of B-cell lymphoma. More recently, ICER have released their evidence report of BCMA-targeted CAR T-cell therapy for the treatment of multiple myeloma. The incremental cost-effectiveness ratio for idecabtagene vicleucel compared with current treatment options for heavily refractory multiple myeloma was approximately $319,000/QALY. ICER suggested that idecabtagene vicleucel would meet a WTP threshold of $100,000/QALY if its list price were reduced to around $200,000. The panel concluded that idecabtagene vicleucel was likely to provide small to substantial net health benefits over standard of care.41
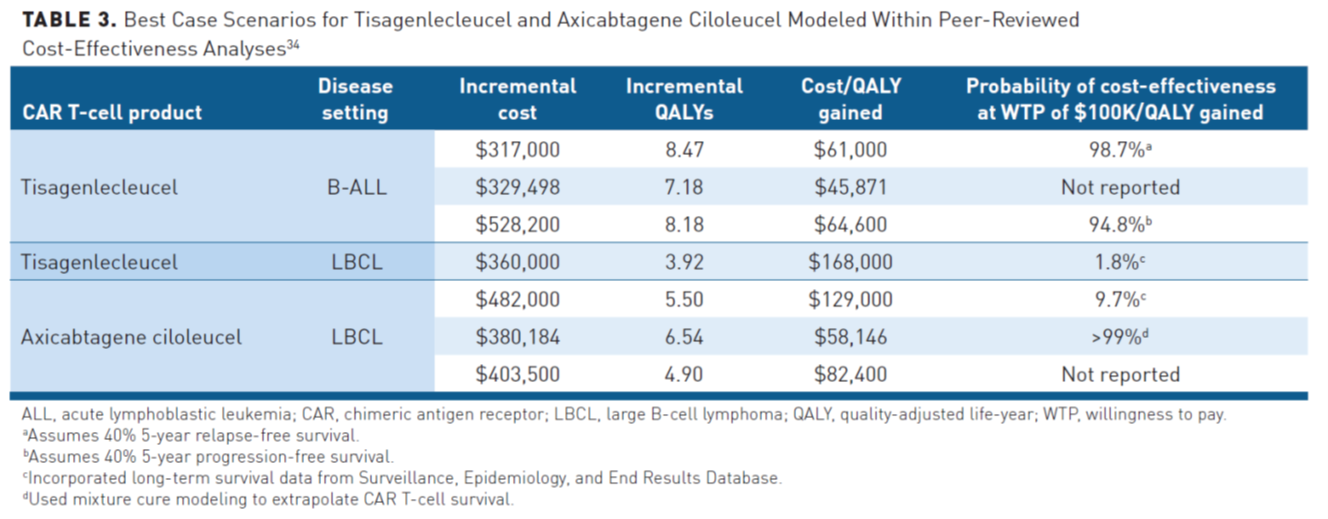
Overall, cost-effectiveness analyses suggest that CAR T-cell therapies could have significant value at their current price, although it should be noted that the wholesale acquisition cost (WAC) of axicabtagene ciloleucel has increased since these studies were published. However, CAR T-cell therapies must result in a substantial increase in overall survival compared with standard of care to achieve acceptable value.34 There are a number of limitations to cost-effectiveness analyses; notably, current assessments of cost-effectiveness are based on limited data related to long-term outcomes (with a median follow-up of <28 months), which can significantly impact their accuracy. Other limitations include their reliance on modeling, differing assumptions about the long-term benefits of CAR T-cell therapy and calculations of total costs, static QALY measurements, and ambiguous WTP thresholds. Furthermore, comparing CAR T-cell therapy to the standard of care does not capture real-world treatment patterns, in which patients may already have been treated with standard of care or may go on to receive additional therapies that add to the cost. Nor do they consider the affordability of these therapies and their impact on healthcare budgets, or the different perspectives and priorities of payers and providers in assessing the value of a therapy. The focus of clinicians is typically centered on the individual patient, while institutions and payers are likely more concerned with providing the most benefit to the greatest number of patients. In a real-world scenario, numerous complicating factors can create added financial pressures for payers, which can lessen the long-term value of a treatment. For example, plan members may change insurers over time or, for small employer groups, expensive therapies could negatively impact the health benefits available to other patients in the group, which can lessen the long-term value of a treatment.34,42 ICER also performs budget impact assessments that measure affordability and assess how a treatment will affect healthcare budgets. ICER reported that only 38% of adult patients with LBCL could be treated with CAR T-cell therapy in a given year without crossing the budgetary impact threshold at the then list price of $373,000. Similarly, only 43% of eligible patients with relapsed/refractory multiple myeloma could be treated with CAR T-cell therapy within 5 years before crossing the budgetary impact threshold at the current list price.40,41
Comprehension of the total cost of care and value is also important in overcoming the sticker shock of CAR T-cell therapy when compared with the available alternatives. Although CAR T-cell therapy has the most expensive WACs, other therapies may require repeat dosing, increasing the total cost of care, and are less effective, thus having lower long-term value. The approximate cost for 1 cycle of blinatumomab for the treatment of adult and pediatric patients with B-cell ALL is between $97,000 and $107,000, but patients may receive up to 9 cycles during a full treatment course, leading to a total cost for drug acquisition of close to $1 million.43 Furthermore, blinatumomab and inotuzumab are approved in the maintenance setting and patients have already undergone expensive allogeneic stem cell transplant.
Managing Costs and Improving Access
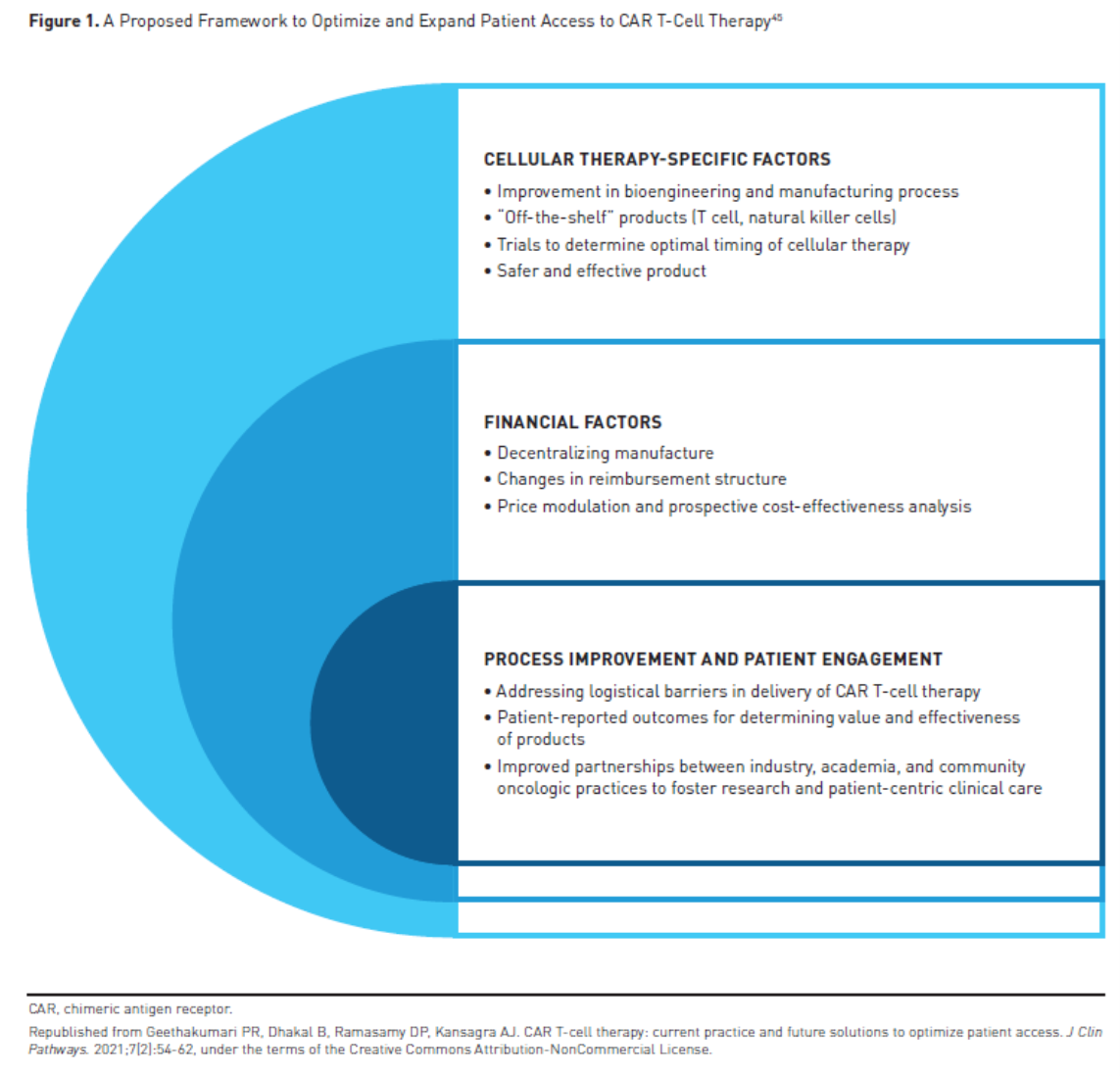
As the demand for CAR T-cell therapy increases, there is a growing need to address the financial challenges and improve access, and there are a number of potential solutions being explored. Barriers to use of CAR T-cell therapy include prior authorization, manufacturing waiting time, toxicity, out-of-pocket costs, geographic access, and others.44 Next-generation CAR T-cell therapies with improved safety and efficacy profiles, manufacturing improvements, and innovative reimbursement strategies may help to improve access (Figure 145).
Site of Care
Although CAR T-cell therapy is currently predominantly delivered in the inpatient setting, there is a growing trend toward outpatient administration, particularly as CAR T-cell products with reduced toxicity reach the market and as experience with managing toxicities grows.19,21 Clinical trials have begun to examine the feasibility of administering CAR T-cell therapy in the outpatient setting. In an analysis of patients with B-cell lymphoma treated with lisocabtagene maraleucel in the outpatient setting in the pivotal TRANSCEND NHL 001 trial and 2 phase 2 trials, 43% of patients remained outpatient. Among patients who required hospitalization, the median time to hospitalization was 5 days, only 3 patients were admitted within 3 days of infusion, and just 2 patients required ICU-level care.46 According to a single-center report on hospitalization patterns with tisagenlecleucel, only about one-third of patients receiving outpatient infusion were subsequently admitted to the hospital, with a median time to hospitalization of 5 days.47
Although outpatient administration can reduce costs, many patients are still subsequently admitted to the hospital due to toxicity. This is particularly important for the Medicare patient population as, although CAR T-cell therapy is covered under Medicare Part B, if patients are admitted to the hospital within Medicare’s 3-day payment window then the outpatient costs will be bundled with the claim for an inpatient stay and the provider loses the ability to claim Part B benefits. Notably, the site of care influences the cost savings from outpatient administration, with the greatest benefit when CAR T-cell therapy is administered at a nonhospital-affiliated site. Hospital outpatient administration is unlikely to benefit payers because it is the costliest site of service for Part B drugs billed under the medical benefit.48 According to a recent cost modeling analysis, outpatient administration of CAR T-cell therapy (in the setting of a nonacademic specialty oncology network) was associated with a greater than 40% (>$30,000) reduction in total costs.19 One limitation of this model is the assumption of equal outcomes to those seen in academic hospital-affiliated clinics. Currently, CAR T-cell therapies are largely restricted to clinics with an established expertise in treating these patients and thus we may not see nonacademic hospital-affiliated clinics caring for these patients in the near future. There are many critical issues that need to be addressed to increase the feasibility of outpatient administration, including the challenge of coordination of care, increasing the availability of resources, ensuring adequate patient and caregiver education, and improving toxicity management.
Reimbursement Strategies
The current payment model for CAR T-cell therapy in the United States results in a significant reimbursement gap. Medicare fee-for-service beneficiaries present a particular challenge, with the current reimbursement model resulting in substantial losses for providers. CMS first announced national coverage of CAR T-cell therapy in 2019; they allotted CAR T-cell therapy the same Medicare Severity Diagnosis Related Group (MS-DRG) code as autologous bone marrow transplantation but included a New Technology Add-on Payment (NTAP).49 In 2021, CMS assigned inpatient CAR T-cell therapy its own unique code, MS-DRG 018, but eliminated the NTAP, which likely means limited net financial impact to institutions.50
For commercial payers, there is no common payment path. Historically, single-case agreements with the specific payer have been the standard, but this has become unsustainable as the use of CAR T-cell therapy expands.31 Numerous alternative payment and care delivery models are being explored.51 These include outcomes-based pricing arrangements, but these present several critical challenges, including determining the optimum outcome to use. Many payers are interested in pursuing a Centers of Excellence model, which concentrates patient care at institutions with specialist providers who have the most experience with CAR T-cell therapy. An advantage of this model is that there is an existing framework from stem cell transplant specialty networks that could be used as a guide.52 Among other reimbursement strategies are bundled payments—a United Healthcare bundled payment intervention in oncology resulted in more than $33 million in net savings—credit mechanisms, cost-sharing (eg, HealthCoin), managed entry agreement, and milestone-based contracts, although this intervention did not include CAR T-cell therapies.51,53,54
Honing CAR T-Cell Products
CAR T cells are currently primarily manufactured by pharmaceutical companies using autologous T cells, but academic medical centers are well equipped to take over the manufacturing process with Good Manufacturing Practice-grade facilities. Decentralizing the CAR T-cell production process could reduce cost and shorten the time from leukapheresis to infusion but is currently limited by the burdensome regulatory pathway for CAR T-cell therapy. Pharmaceutical companies are also searching for ways to improve CAR T-cell manufacturing technology to ensure cheaper and quicker access to CAR T-cell therapies. A major focus is the development of “off-the-shelf” allogeneic CAR T-cell therapies, generated from a donor, but graft-versus-host disease, wherein the transplanted T cells attack healthy tissue causing severe toxicity, presents a significant challenge.45
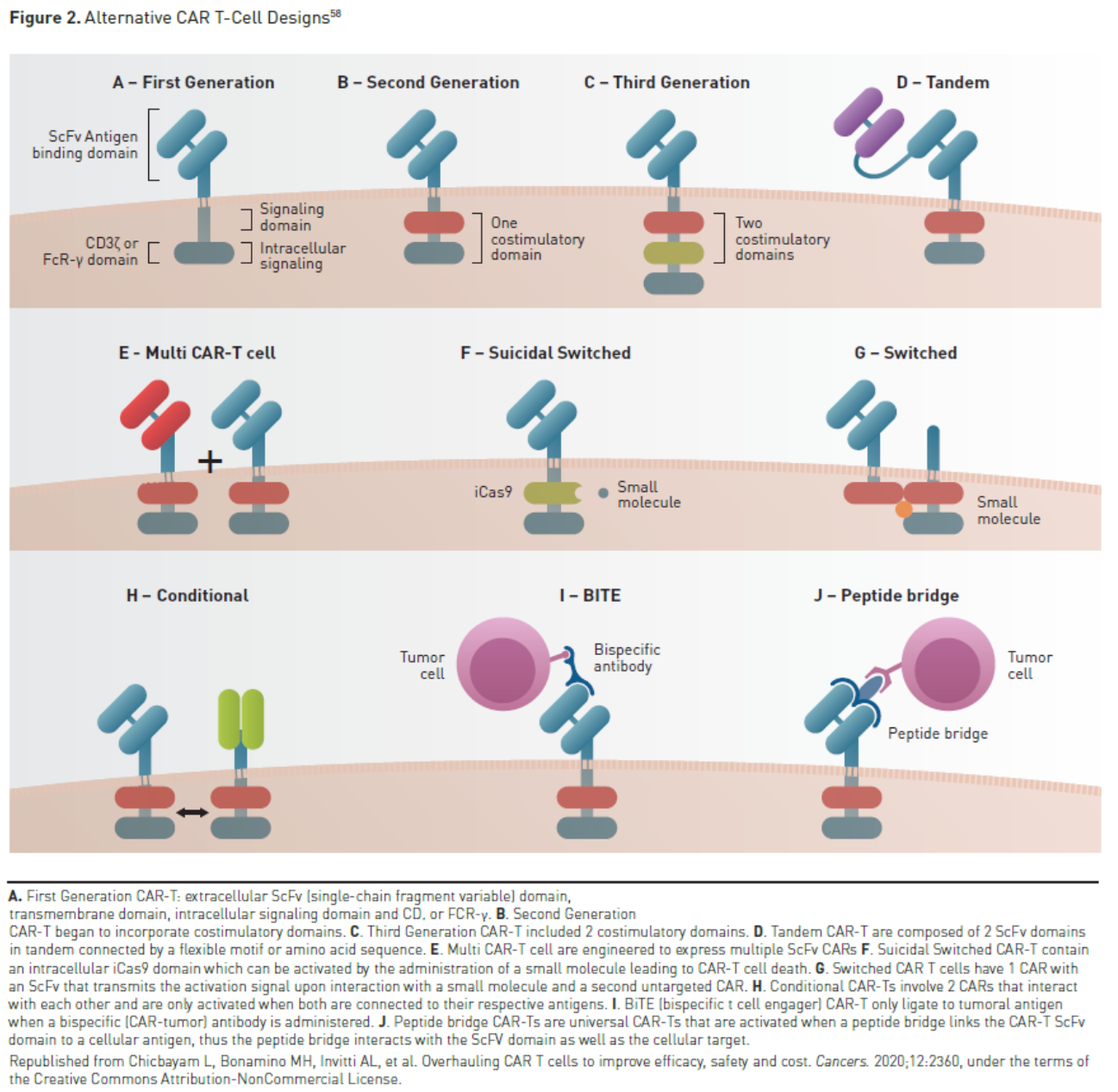
Another central goal is the development of safer and more effective CAR T-cell therapies. A number of strategies for mitigating toxicity are being explored. Disease burden has been shown to be a significant risk factor for severe toxicity and risk-adapted strategies such as debulking therapy to reduce disease burden or reduced CAR T-cell dose in patients with higher disease burden could prove useful. Risk-adapted dosing has been shown to reduce the rates of severe CRS in patients with B-cell ALL and high marrow blast counts.55,56 Prophylactic treatment of toxicity is also being investigated; prophylactic tocilizumab has been shown to reduce the rates of severe CRS in patients treated with CAR T-cell therapy.57 A number of next-generation CAR designs are in various stages of clinical development that aim to improve safety or efficacy, such as switchable CARs, armored CARs, tandem CARs, and universal CARs (Figure 258).For now and in the near future, all CAR T-cell products are targeted to treat hematologic malignancies. Work is currently underway to translate this experience into the treatment of solid tumor malignancies. If CAR T-cell therapy expands into the treatment of solid tumors, it will greatly expand the utilization of CAR T-cell therapies based on the much larger number of potential patients.
Conclusions
CAR T-cell therapy is rapidly expanding, with new FDA-approved indications, an increasing number of centers accredited to administer them, and a global market predicted to be worth many billions of dollars within the next 5 years. The costs associated with CAR T-cell therapy present a major obstacle to effective implementation and patient access. These costs extend well beyond the drug acquisition cost, with total cost of care potentially reaching $1 million or more, particularly among patients who experience toxicity. Cost-effectiveness analyses suggest CAR T-cell therapies could have significant value at their current price; however, budget impact analyses show that fewer than half of eligible patients could be treated before ICER’s budget impact threshold is crossed. A growing trend toward outpatient administration could help to reduce costs and improve value, but only if CAR T-cell therapies become eligible to be administered at nonhospital-affiliated centers and only if Medicare patients can avoid inpatient admission that triggers the 3-day rule. The current single-case agreement model that predominates in reimbursement is unsustainable and a number of alternative strategies are under investigation.
Author affiliation: Rebecca Borgert, PharmD, BCOP, is senior director, Oncology Clinical Strategy and Innovation, Magellan Rx Management, Gainesville, FL.
Funding source: This activity is supported by educational grants from Bristol Myers Squibb; Kite Pharma, Inc; and Novartis Pharmaceuticals Corporation.
Author disclosure: Dr Borgert has the following relevant financial relationships with commercial interests to disclose: Consultant – Seagen; Advisory boards – Pfizer, Sanofi; Spouse – Employee: Melinta Therapeutics.
Authorship information: Concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
Address correspondence to: rjborgert@magellanhealth.com
Medical writing and editorial support provided by: Jane de Lartigue, PhD
References
1. FDA. 2017 Biological license application approvals. Updated April3, 2018. Accessed June 11, 2021. www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2017-biological-license-application-approvals
2. FDA. 2018 Biological license application supplement noteworthy approvals. Updated January 31, 2019. Accessed July 29, 2021. www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2018-biological-license-application-supplement-noteworthy-approvals
3. FDA. 2021 Biological license application supplement noteworthy approvals. Updated June 25, 2021. Accessed July 12, 2021. www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2021-biological-license-application-supplement-noteworthy-approvals
4. FDA. 2020 Biological license application approvals. Updated August 20, 2020. Accessed June 11, 2021. www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2020-biological-license-application-approvals
5. Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; June 2021. Accessed July 27, 2021. www.novartis.us/sites/www.novartis.us/files/kymriah.pdf
6. Yescarta. Prescribing information. Kite Pharma; April 2021. Accessed July 7, 2021. www.gilead.com/-/media/files/pdfs/medicines/oncology/yescarta/yescarta-pi.pdf
7. Breyanzi. Prescribing information. Bristol Myers Squibb; February 2021. Accessed July 6, 2021. https://packageinserts.bms.com/pi/pi_breyanzi.pdf
8. Tecartus. Prescribing information. Kite Pharma; February 2021. Accessed July 7, 2021. www.gilead.com/-/media/files/pdfs/medicines/oncology/tecartus/tecartus-pi.pdf
9. Abecma. Prescribing information. Bristol Myers Squibb; March 2021. Accessed July 6, 2021. https://packageinserts.bms.com/pi/pi_abecma.pdf
10. IPD Analytics. WAC Pricing for CAR T cells. Accessed June 11, 2021. www.ipdanalytics.com
11. Sternberg A. Application for brexucabtagene autoleucel in B-cell ALL submitted to FDA for consideration. April 9, 2021. Accessed May 30, 2021. www.cancernetwork.com/view/application-for-brexucabtagene-autoleucel-in-b-cell-all-submitted-to-fda-for-consideration
12. Biospace. Janssen initiates rolling submission of a biologics license application to US FDA for BCMA CAR-T therapy ciltacabtagene autoleucel (cilta-cel) for the treatment of relapsed and/or refractory multiple myeloma. December 21, 2020. Accessed May 30, 2021. www.biospace.com/article/releases/janssen-initiates-rolling-submission-of-a-biologics-license-
application-to-u-s-fda-for-bcma-car-t-therapy-ciltacabtagene-autoleucel-cilta-cel-for-the-treatment-of-relapsed-and-or-refractory-multiple-myeloma
13. May B. Barriers and solutions to expanding access to CAR T-cell therapy. ASCO Daily News. August 9, 2020. Accessed May 5, 2021. https://dailynews.ascopubs.org/do/10.1200/ADN.20.200294/full/#:~:text=More%20than%20600%20active%20CAR,products%20per%20year%20by%202025
14. Caffrey M. Real-world data offer good news for CAR T-cell therapy. Am J Manag Care. December 15, 2020. Accessed May 17, 2021. www.ajmc.com/view/real-world-data-offers-good-news-for-car-t-cell-therapy
15. PR Newswire. Global CAR-T Therapy Market Report 2020: market is expected to stabilize and reach $3,150 million in 2025 - COVID-19 impact and recovery forecast to 2030. February 1, 2021. Accessed May 17, 2021. www.prnewswire.com/news-releases/global-car-t-therapy-market-report-2020-market-is-expected-to-stabilize-and-reach-3-150-million-in-2025---covid-19-impact-and-recovery-forecast-
to-2030--301218802.html
16. Grand View Research. T-cell therapy market size, share & trends analysis report by modality, by therapy (CAR T-cell, tumor-infiltrating lymphocytes), by indication (hematologic malignancies, solid tumors), by region, and segment forecasts, 2021 – 2028. February 2021. Accessed May 17, 2021.
www.grandviewresearch.com/industry-analysis/t-cell-therapy-market
17. Market Research Future. Global CAR T cell therapy market: information by target antigen
(CD19, CD22), by application (diffuse large B-cell lymphoma, acute lymphoblastic leukemia) and region (US, Europe, China and rest of world) - forecast till 2027. March 2021. Accessed May 17, 2021.
www.marketresearchfuture.com/reports/car-t-cell-therapy-market-8102
18. Red Book Online. IBM Corporation; 2021. Accessed May 19, 2021. www.ibm.com/products/micromedex-red-book
19. Lyman G, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Network Open. 2020;3(4):e202072. doi: 10.1001/jamanetworkopen.2020.2072
20. BMT Infonet. Medical centers offering CAR T-cell therapy. Accessed March 9, 2021. www.bmtinfonet.org/transplant-article/medical-centers-offering-car-t-cell-therapy
21. Mahmoudjafari Z, Hawks KG, Hsieh AA, Plesca D, Gatwood KS, Culos KA. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group Survey on Chimeric Antigen Receptor T Cell Therapy Administrative, Logistic, and Toxicity Management Practices in the United States. Biol Blood Marrow Transplant. 2019;25(1):26-33. doi: 10.1016/j.bbmt.2018.09.024
22. Harris AH, Hohmann S, Shippey E, Epting G. Quality and cost outcomes in chimeric antigen receptor T-cell immunotherapy in adult large B-cell cancer patients from the Vizient Clinical Database. Blood Biol Marrow Transplant. 2020;26(3):S316-S317. Abstract 486.
23. Brown AR, Rajendram P, Herr M, et al. Chimeric antigen receptor (CAR) cell patients admitted to the ICU: the CAR-ICU Initiative Experience. Crit Care Med. 2021;49(1):232.
24. Jain MD, Jacobs MT, Nastoupil LJ, et al. Characteristics and outcomes of patients receiving bridging therapy while awaiting manufacture of standard of care axicabtagene ciloleucel CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: results from the US Lymphoma CAR-T Consortium. Blood. 2019;134(suppl_1):245.
25. Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T – and a side order of IgG to go? – immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019;38:100596. doi: 10.1016/j.blre.2019.100596
26. Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4(7):994-996. doi: 10.1001/jamaoncol.2018.0977
27. Centers for Medicare & Medicaid Services (CMS) Healthcare Common Procedure Coding System (HCPCS) application summaries and coding decisions fourth quarter, 2020 coding cycle for drug and biological products. Accessed June 16, 2021. www.cms.gov/files/document/2020-hcpcs-application-summary-quarter-4-2020-drugs-and-biologics.pdf
28. Dustman R. Take 5: Medicare News Flash – May 2021. Accessed June 16, 2021. www.aapc.com/blog/73840-take-5-medicare-news-flash-may-2021
29. Komanduri K. ASBMT Letter Re: CMS issuance of code Q2041. February 9, 2018. Accessed May 24, 2021. https://higherlogicdownload.s3.amazonaws.com/ASBMT/43a1f41f-55cb-4c97-9e78-
c03e867db505/UploadedImages/ASBMT_Inquiry_Q2041_Yescarta_2_9_2018.pdf
30. Hagen T. CAR T-cell therapy: the sticker price is just for openers. OncologyLive. 2018;19:12. Accessed May 24, 2021. www.onclive.com/view/car-tcell-therapy-the-sticker-price-is-just-for-openers
31. Alexander M, Culos K, Roddy J, et al. Chimeric antigen receptor T-cell therapy: a comprehensive review of clinical efficacy, toxicity, and best practices for outpatient administration. Transplant Cell Ther. 2021;27(7):558-570. doi: 10.1016/j.jtct.2021.01.014
32. de Lima Lopes G, Nahas GR. Chimeric antigen receptor T cells, a savior with a high price. Chinese Clin Oncol. 2018;7(2):21. doi: 10.21037/cco.2018.04.02
33. Amorosi D. CAR T-cell therapy total cost can exceed $1.5 million per treatment. May 29, 2019. Accessed February 24, 2021. www.healio.com/news/hematology-oncology/20190529/car-tcell-therapy-total-cost-can-exceed-15-million-per-treatment
34. Fiorenza S, Ritchie DS, Ramsey SD, Turtle CJ, Roth JA. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant. 2020;55(9):1706-1715. doi: 10.1038/s41409-020-0956-8
35. Lin JK, Lerman BJ, Barnes JI, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192-3202. doi: 10.1200/JCO.2018.79.0642
36. Sarkar RR, Gloude NJ, Schiff D, Murphy JD. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719-726. doi: 10.1093/jnci/djy193
37. Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172(12):1161-1168. doi: 10.1001/jamapediatrics.2018.2530
38. Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21(12):1238-1245. doi: 10.1080/13696998.2018.1529674
39. Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2(2):e190035. doi: 10.1001/jamanetworkopen.2019.0035
40. Institute for Clinical and Economic Review. Leukemia and lymphoma. March 2018. Accessed March 11, 2021. https://icer.org/assessment/leukemia-and-lymphoma-2018
41. Institute for Clinical and Economic Review. Anti B-cell maturation antigen CAR T-cell and antibody drug conjugate therapy for heavily pretreated relapsed and refractory multiple myeloma. April 2021. Accessed May 28, 2021. https://icer.org/wp-content/uploads/2020/10/ICER_Multiple-Myeloma_Evidence-Report_040521-1.pdf
42. Whittington MD, McQueen RB, Campbell JD. Valuing chimeric antigen receptor T-cell therapy: current evidence, uncertainties, and payment implications. J Clin Oncol. 2020;38(4):359-366. doi: 10.1200/JCO.19.01558
43. Drawdy L, Jones LA, Hall PD. Blinatumomab: a step forward in the treatment of B-cell precursor acute lymphoblastic leukemia. J Hematol Oncol Pharm. 2019;9(2):38-46.
44. Kansagra A, Farnia S, Majhail N. Expanding access to chimeric antigen receptor T-cell therapies: challenges and opportunities. Am Society Clin Oncol Educ Book. 2020;(40):e27-e34. doi: 10.1200/EDBK_279151
45. Geethakumari PR, Dhakal B, Ramasamy DP, Kansagra AJ. CAR T-cell therapy: current practice and future solutions to optimize patient access. J Clin Pathways. 2021;7(2):54-62. doi: 10.25270/jcp.2021.03.00002
46. Bachier CR, Palomba ML, Abramson JS, et al. Outpatient Treatment with Lisocabtagene Maraleucel (liso-cel) in 3 Ongoing Clinical Studies in Relapsed/Refractory (R/R) Large B Cell Non-Hodgkin Lymphoma (NHL), Including Second-Line Transplant Noneligible (TNE) Patients: Transcend NHL 001, Outreach, and PILOT. Biol Blood Marrow Transplant. 2020;26(3):S25-S26. doi: 10.1016/j.bbmt.2019.12.093
47. Dwivedy Nasta S, Namoglu EC, Hughes ME, et al. Hospitalization patterns with commercial CAR T-cell therapy: a single institution experience. Blood. 2019;134(suppl_1):3240-3240. doi: 10.1182/blood-2019-130650
48. Marwood Group. Navigating provider challenges to growth of the CAR T-cell therapy space. 2020. Accessed July 7, 2021. www.marwoodgroup.com/wp-content/uploads/2020/01/Marwood-Navigating-Provider-Challenges-to-Growth-of-The-CAR-T-Cell-Therapy-Space.pdf
49. Centers for Medicare & Medicaid Services. Decision memo for chimeric antigen receptor (CAR) T-cell therapy for cancers (CAG-00451N). August 7, 2019. Accessed May 28, 2021. www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=291
50. Centers for Medicare & Medicaid Services. FY 2021 IPPS Final Rule Home Page. January 15, 2021. Accessed May 28, 2021. www.cms.gov/medicare/acute-inpatient-pps/fy-2021-ipps-final-rule-home-page
51. Aviki EM, Schleicher SM, Mullangi S, Matsoukas K, Korenstein D. Alternative payment and care-delivery models in oncology: a systematic review. Cancer. 2018;124(16):3293-3306. doi: 10.1002/cncr.31367
52. MIT NEWDIGS FoCUS Project. Role of Centers of Excellence (COE) Networks in the Delivery of Curative Cellular Therapies in Oncology. September 21, 2018. Accessed May 28, 2021. https://newdigs.mit.edu/sites/default/files/FoCUS%20Research%20Brief%202018F209v026_0.pdf
53. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326. doi: 10.1200/JOP.2014.001488
54. Basu A. Financing cures in the United States. Exp Rev Pharmacoecon Outcomes Res. 2015;15(1):1-4. doi: 10.1586/14737167.2015.990887
55. Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433-444. doi: 10.1200/EDBK_238691
56. Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183(3):364-374. doi: 10.1111/bjh.15644
57. Caimi PF, Sharma A, Rojas P, et al. CAR-T therapy for lymphoma with prophylactic tocilizumab: decreased rates of severe cytokine release syndrome without excessive neurologic toxicity. Presented at the 62nd ASH Annual Meeting and Exposition. December 5-8, 2020. Abstract 738.
58. Chicbayam L, Bonamino MH, Invitti AL, et al. Overhauling CAR T cells to improve efficacy, safety and cost. Cancers. 2020;12:2360. doi: 10.3390/cancers12092360
